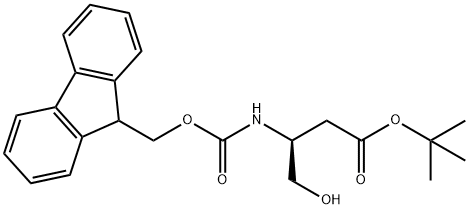
FMOC-ASPARTIMOL(OTBU) synthesis
- Product Name:FMOC-ASPARTIMOL(OTBU)
- CAS Number:133565-45-4
- Molecular formula:C23H27NO5
- Molecular Weight:397.46
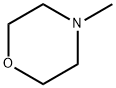
109-02-4
512 suppliers
$10.00/5ml
71989-14-5
475 suppliers
$5.00/1g

543-27-1
265 suppliers
$10.00/5g

133565-45-4
77 suppliers
$48.00/250mg
Yield:-
Reaction Conditions:
with sodium borohydrid in tetrahydrofuran;methanol;ammonium chloride
Steps:
1.1 Step 1:
Step 1: tert-Butyl (3S)-3-[(9H-9-fluorenylmethoxy)carbonyl]amino-4-hydroxybutanoate To a solution of N-Fmoc-L-aspartic acid b-tert-butyl ester (19.0 g, 46.2 mmol) in 300 mL of tetrahydrofuran (THF) at -78° C. was added N-methyl morpholine (NMM, 5.9 mL, 53.3 mmol) followed by isobutyl chloroformate (IBCF, 6.9 mL, 53.3 mmol). After 10 minutes this mixture was warmed to 0° C. for 40 minutes and then recooled to -78° C. A suspension of sodium borohydride (3.85 g, 102 mmol) in 25 mL of methanol was added and the mixture was stirred at -78° C. for 2 h. The reaction was quenched into 400 mL saturated aqueous ammonium chloride and extracted with ethyl acetate (4*100 mL). The combined organic layers were washed with brine and dried over anhydrous magnesium sulfate, filtered and concentrated. The residue was purified on silica gel (50% ethyl acetate/hexane) to give the desired product: 1H NMR (400 MHz, CD3COCD3) d 7.85 (d, 2H, J=7.30 Hz), 7.67 (d, 2H, J=7.37 Hz), 7.40 (t, 2H, J=7.30 Hz), 7.30 (t, 2H, J=7.30 Hz), 6.32 (brd, 1H), 4.40-4.15 (m, 3H), 4.10-3.98 (m, 1H), 3.92 (t, 1H), 3.65-3.48 (m, 2H), 2.60 (dd, 1H, J=6.24, 16.80 Hz), 2.41 (dd, 1H, J=6.30, 16.91 Hz), 1.40 (5, 9H).
References:
Merck Frosst Canada & Co. US6552168, 2003, B1

71989-14-5
475 suppliers
$5.00/1g

133565-45-4
77 suppliers
$48.00/250mg
86061-01-0
108 suppliers
$41.70/1g

133565-45-4
77 suppliers
$48.00/250mg
![Butanoic acid, 3-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-4-oxo-, 1,1-dimethylethyl ester, (3S)-](/CAS/20210111/GIF/146803-45-4.gif)
146803-45-4
6 suppliers
inquiry

133565-45-4
77 suppliers
$48.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

133565-45-4
77 suppliers
$48.00/250mg