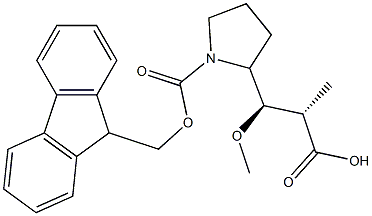
Fmoc-Dap synthesis
- Product Name:Fmoc-Dap
- CAS Number:863971-41-9
- Molecular formula:C24H27NO5
- Molecular Weight:409.47
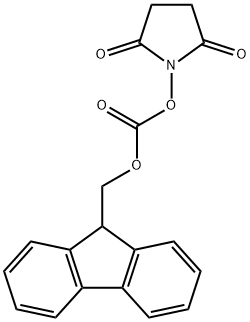
82911-69-1
604 suppliers
$7.00/25g
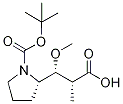
120205-50-7
116 suppliers
$91.00/250mg

863971-41-9
7 suppliers
inquiry
Yield: 3.4 g
Reaction Conditions:
Stage #1:N-Boc-(2R,3R,4S)-dolaproine with hydrogenchloride at 20; for 3 h;Inert atmosphere;
Stage #2:N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide with sodium carbonate in 1,2-dimethoxyethane
Steps:
1 Step 1. Synthesis of (2R,3R)-3- {(2S)- 1 -[(9H-fluoren-9-ylmethoxy)carbonyl]pyrrolidin-2- yl} -3-methoxy-2-methylpropanoic acid (119)
To a stirring solution of 11 (2.4 g, 8.4 mmol, 1.0 eq.) in 10 mL of dioxane under nitrogen, 4M HCl in dioxane(20 mL, 80 mM, 10 eq.) was added. The reaction was allowed to stir at room temperature for 3 hours before being concentrated in vacuo and placed underneath high vacuum. Crude material was then dissolved with 30 mL of 10% Na2C03. This solution was then added to a stirring solution of 1- {[(9H- fluoren-9-ylmethoxy)carbonyl]oxy}pyrrolidine-2,5-dione (2.96 g, 8.77 mmol, 1.05 eq.) in 30 mL of DME. Reaction was allowed to stir at room temperature until TLC (20% methanol/40% ethyl acetate/40% heptanes) indicated the consumption of Boc de-protected starting material. The reaction was concentrated in vacuo to a smaller volume, washed twice with ether, acidified to pH 2 using concentrated HCl and then extracted three times with a solution of 90% dichloromethane 10%methanol. The organics where washed with saturated sodium bicarbonate and brine before being dried over sodium sulfate, filtered, and concentrated in vacuo to a brown solid 119 (3.4 g, quant.). LC-MS (Protocol Q): m/z 410.0 [M+H+], retention time = 1.81 minutes.
References:
PFIZER INC.;DOROSKI, Matthew David;MADERNA, Andreas;O'DONNELL, Christopher John;SUBRAMANYAM, Chakrapani;VETELINO, Beth Cooper;DUSHIN, Russell George;STROP, Pavel;GRAZIANI, Edmund, Idris WO2013/72813, 2013, A2 Location in patent:Page/Page column 173

82911-69-1
604 suppliers
$7.00/25g

863971-41-9
7 suppliers
inquiry
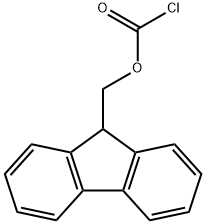
28920-43-6
530 suppliers
$5.00/5g

120205-50-7
116 suppliers
$91.00/250mg

863971-41-9
7 suppliers
inquiry
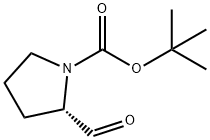
69610-41-9
250 suppliers
$9.00/1g

863971-41-9
7 suppliers
inquiry
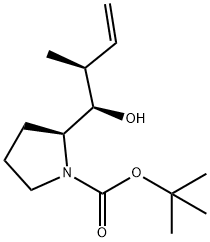
159173-40-7
8 suppliers
inquiry

863971-41-9
7 suppliers
inquiry