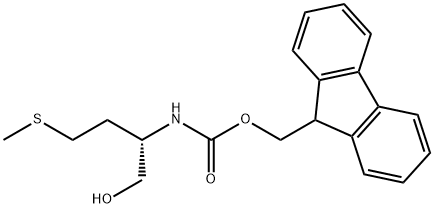
FMOC-L-METHIONINOL synthesis
- Product Name:FMOC-L-METHIONINOL
- CAS Number:133565-47-6
- Molecular formula:C20H23NO3S
- Molecular Weight:357.47
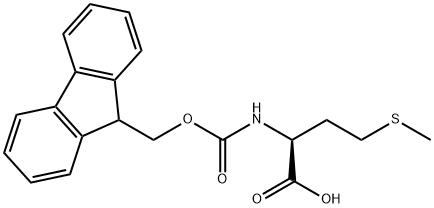
71989-28-1
417 suppliers
$6.41/1mmol

133565-47-6
18 suppliers
inquiry
Yield:133565-47-6 89%
Reaction Conditions:
Stage #1: N-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-methioninewith 4-methyl-morpholine;isobutyl chloroformate in 1,2-dimethoxyethane at -5 - 10;
Stage #2: with sodium tetrahydroborate in water;
Steps:
7 Example 7: Reduction of Chiral N-Protected Amino Acids to N-protected Amino Alcohols - Formula 6 (See: Fig 20)
General procedure: By way of an example, the procedure of Rodriquez et al. (Ref. C-21 ) was followed to produce both the D- and L-enantiomers of Fmoc methionine. In each case, 25 mmol of N-Fmoc methionine was dissolved/suspended in 25 ml. of 1 ,2-dimethoxyethane ("DME") and this solution was cooled in an ice/salt bath to about -5-10°C (See: Table 7B). Then, a slight excess (25.5-26 mmol) of NMM was added and allowed to stir for about 1 -3 minutes before isobutyl chloroformate (25.5-26 mmol) was added. After a few minutes of reacting, the reaction was filtered to remove the N-methylmorpholine hydrochloride. The filter cake was then washed several times with 5 mL portions of DME. To the filtrate was added a solution of 39-40 mmol of sodium borohydride dissolved in 13 mL deionized water with mixing and then immediately thereafter (400-650ml_) of deionized water was added to produce a white solid. This white solid was collected by vacuum filtration and the cake washed with water and then hexanes. The product was dried under high vacuum. According to Rodriquez, this procedure is generally applicable to the other amino acids. Indeed, this general procedure was also shown to be effective to produce both L- and D- enantiomers of suitably protected serine (See: Table 7B).
References:
WO2018/175927,2018,A2 Location in patent:Paragraph 00312-00315
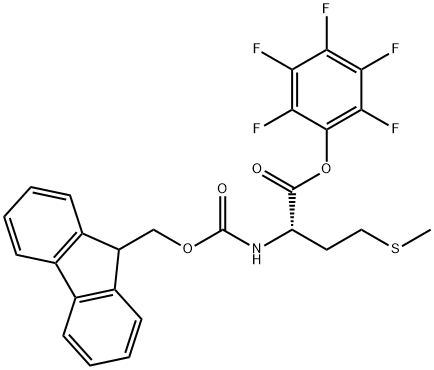
86060-94-8
82 suppliers
$24.19/1g

133565-47-6
18 suppliers
inquiry