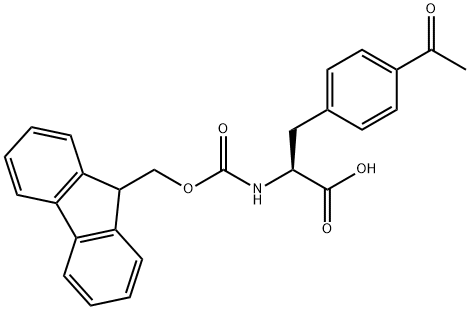
FMOC-L-PHE(4-COCH3) synthesis
- Product Name:FMOC-L-PHE(4-COCH3)
- CAS Number:204716-07-4
- Molecular formula:C26H23NO5
- Molecular Weight:429.46
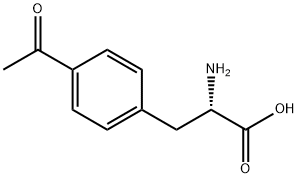
122555-04-8
61 suppliers
$61.60/100MG
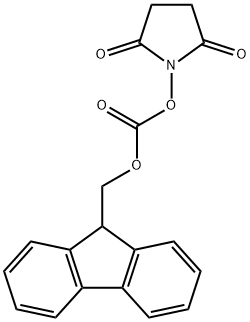
82911-69-1
607 suppliers
$7.00/25g

204716-07-4
35 suppliers
inquiry
Yield:204716-07-4 98%
Reaction Conditions:
with sodium hydrogencarbonate in water;acetone; for 3 h;
Steps:
Synthesis of enatiopure N-FmocpAcF (and mAcF).
This compound was synthesized according to the pocedue described in FIGURE 5A. Racemic p-acetylphenylatanine (1 g, 4.83 mmol, 1 eq) was dissolved in acetic acid (20 mL) to which acetic anhydride was added (4.52 mL, mniol, 10 eq). The reaction was stirred at room temperature for 2 h, followed by removal of the solvent by evaporation. The crude product was redissolved in phosphate buffer (50 mL) containing 1 mM CoCl26H2O at pH 8.0, followed by addition of acylase 1 (500 mg). The reaction was stirred at 37°C for 24 h with occasional adjustment of the pH to 8.0 with LIOH. The reaction mixture was heated to 60°C for 5 mm, cooled to room temperature and filtered through celite. The filtrate was acidified to pH 3 using HCI and then extracted with EtOAc. The aqueous layer was lyophilized and used as crude product (410 mg L-enantiomer, yield - 82%) for the next reaction. L-p-acetyl-phenylalanine (410 mg, 1.98 mmol, I eq) was dissolved in a water/acetone mixture (1:1, v/v) to which NaHCO3 (332.6 mg, 3.96 mmol, 2 eq) was added. Fmoc-OSu (735.4 mg, 2.18 mmol, 1.1 eq) was dissolved in acetone and added to the reaction mixture portion wise over the course of 3 h. Upon completion of the reaction, acetone was remove by evaporation and the aqueous layer acidified with acetic acid to pH 3 followed by EtOAc extraction. The organic layers were combined, dried over sodium sulfate and evaporated. The crude product was purified with flash column chromatography and solvent system hexanes:EtOAc:AcOH (10:9:1) to yield pure Fmoc-p-acetyl-L-phenylalanirie (832.7 mg, 98%). 1H NMR (500 MHz, CD3OD), 52.57 (s, 3H), 3.22-3.41 (m, 2H), 4.45 (t, 1H), 7.3-7.55 (m, 8H),7.88 (d, 2H), 7.98 (d, 2H). MS (ESI) calculated for C26H23N05 [M+HJ: m/z 429.16, found429.4. An identical procedure was applied to obtain Fmoc-m-acetyl-L-phenylalanine.
References:
WO2015/153761,2015,A2 Location in patent:Paragraph 00252
1403957-00-5
0 suppliers
inquiry

204716-07-4
35 suppliers
inquiry
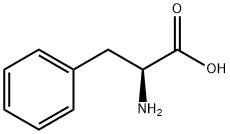
63-91-2
876 suppliers
$10.00/100G

204716-07-4
35 suppliers
inquiry
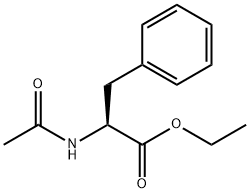
2361-96-8
97 suppliers
$7.00/250mg

204716-07-4
35 suppliers
inquiry