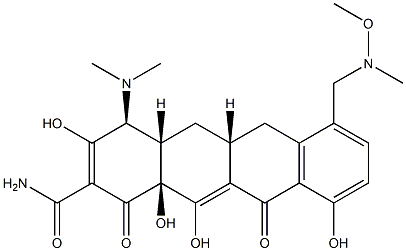
Fosravuconazole bis(L-lysine synthesis
- Product Name:Fosravuconazole bis(L-lysine
- CAS Number:1035654-66-0
- Molecular formula:C24H29N3O8
- Molecular Weight:487.5
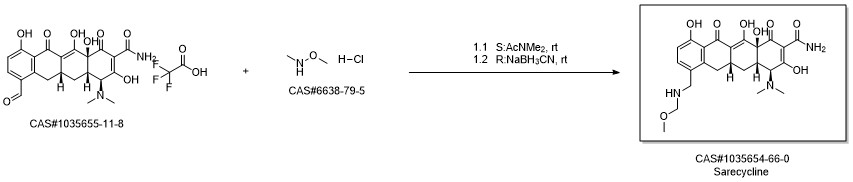
Reference: Assefa, Haregewin; Bhatia, Beena; Draper, Michael; Honeyman, Laura; Molnar, Dennis P.; Kim, Oak K. Preparation of substituted tetracycline compounds for treatment of inflammatory skin disorders. Assignee Paratek Pharmaceuticals, Inc., USA. CA 2892739. (2008).
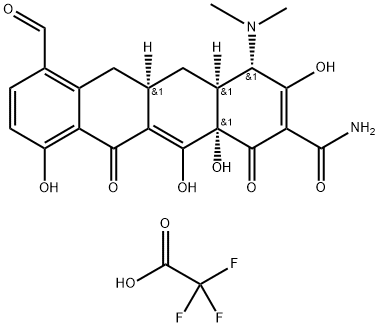
1035655-11-8
3 suppliers
inquiry

6638-79-5
553 suppliers
$6.00/25g

1035654-66-0
31 suppliers
inquiry
Yield:1035654-66-0 385 mg
Reaction Conditions:
Stage #1:7-formylsancycline TFA salt;N,O-dimethylhydroxylamine*hydrochloride in N,N-dimethyl acetamide at 20; for 0.166667 h;Inert atmosphere;
Stage #2: with sodium cyanoborohydride in N,N-dimethyl acetamide for 0.0833333 h;
Steps:
Synthesis of (4S,4aS,5aR,12aS)-4-dimethylamino-3,10,12,12a-tetrahydroxy-7-[(methoxy(methyl)amino)-methyl]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide ("the free base")
Synthesis of (4S,4aS,5aR,12aS)-4-dimethylamino-3,10,12,12a-tetrahydroxy-7-[(methoxy(methyl)amino)-methyl]-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide ("the free base")
A solution of 7-formylsancycline TFA salt (2.23 g) and N,O-dimethylhydroxylamine hydrochloride (780 mg) in N,N-dimethylacetamide (15 mL) was stirred for 10 minutes at room temperature under argon atmosphere.
To this solution was added sodium cyanoborohydride (302 mg).
The solution was stirred for 5 minutes and monitored by LC-MS.
The reaction mixture was poured into diethyl ether, and the resulting precipitates were collected by filtration under vacuum.
The crude product was purified by prep-HPLC using a C18 column (linear gradient 10-40% acetonitrile in 20 mM aqueous triethanolamine, pH 7.4).
The prep-HPLC fractions were collected, and the organic solvent (acetonitrile) was evaporated under reduced pressure.
The resulting aqueous solution was loaded onto a clean PDVB SPE column, washed with distilled water, then with a 0.1 M sodium acetate solution followed by distilled water.
The product was eluted with acetonitrile.
The eluent was concentrated under reduced pressure, 385 mg was obtained as free base.
References:
Paratek Pharmaceuticals, Inc.;Warmer Chilcott Company, LLC;Coulter, Catherine;Johnston, Sean M.;Seyedi, Farzaneh;deVries, Tina M. US2013/302442, 2013, A1 Location in patent:Paragraph 0098; 0099
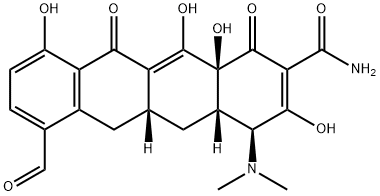
1035655-10-7
1 suppliers
inquiry

6638-79-5
553 suppliers
$6.00/25g

1035654-66-0
31 suppliers
inquiry
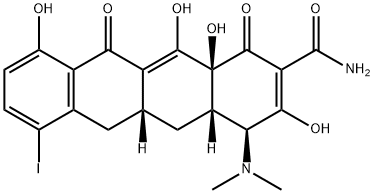
113164-67-3
6 suppliers
inquiry

1035654-66-0
31 suppliers
inquiry
