
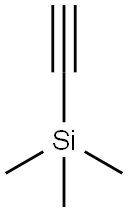
FURAN-3-YLETHYNYLTRIMETHYLSILANE synthesis
- Product Name:FURAN-3-YLETHYNYLTRIMETHYLSILANE
- CAS Number:465521-19-1
- Molecular formula:C9H12OSi
- Molecular Weight:164.28
75-77-4
564 suppliers
$5.04/10

465521-19-1
28 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
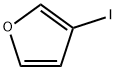
Stage #1: 3-(2,2-dibromovinyl)furanwith n-butyllithium in tetrahydrofuran;hexane at -78 - 20; for 1.5 h;
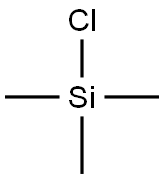
Stage #2: chloro-trimethyl-silane in tetrahydrofuran;hexane at -78 - 20; for 19 h;
Steps:
Furan-3-ylethynyl-trimethyl-silane (11)
To a stirred and cooled (-78 C) solution of the DIBROMOFURAN 10 (2.0 g, 7.9 mmol) IN THF (20 mL) was added 1.6 M n-BuLi in hexane (10. 2 ML, 16.3 mmol) over 3 min. After 1 h the cooling bath bath was removed and the mixture allowed to warm to r. t. Following a further 1.5 h the mixture was cooled back TO-78 C and then TMSC1 (3.0 mL, 23. 8 mmol) was added dropwise. The mixture was allowed to gradually warm up to r. t.. After 19 h the mixture was poured into ice-cold ET2O-SATURATED aqueous NAHC03 solution. The organic layer was separated and the aqueous layer extracted with Et2O (2x). The combined organic solutions were dried (MgS04), filtered and concentrated to afford the crude acetylene 11 (1.83 g) as a yellow oil. This material was used in the next step without purification. 1H NMR (400 MHz; CDC13) inter alia 8 0.23 (s, 9H), 6.44 (m, 1H), 7.34 (m, 1H) and 7.63 (m, 1H).
References:
WO2004/78756,2004,A2 Location in patent:Page 104-105

27607-77-8
489 suppliers
$10.00/5g

51061-85-9
10 suppliers
inquiry

465521-19-1
28 suppliers
$45.00/100mg
29172-20-1
2 suppliers
inquiry
1066-54-2
465 suppliers
$9.00/10g

465521-19-1
28 suppliers
$45.00/100mg