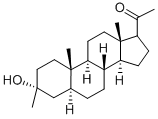
GANAXOLONE synthesis
- Product Name:GANAXOLONE
- CAS Number:38398-32-2
- Molecular formula:C22H36O2
- Molecular Weight:332.52
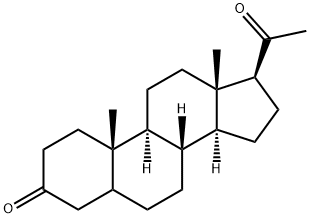
566-65-4
163 suppliers
$50.00/1g
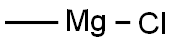
676-58-4
286 suppliers
$15.00/25ml

38398-32-2
75 suppliers
$28.00/1mg
Yield:38398-32-2 91%
Reaction Conditions:
Stage #1: methylmagnesium chloridewith iron(III) chloride;lithium chloride in tetrahydrofuran at -35;
Stage #2: dihydroprogesterone in tetrahydrofuran at -35 - -18; for 3 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran at 25;Product distribution / selectivity;
Steps:
6
EXAMPLE 6.; [0051] A reaction flask is charged with anhydrous lithium chloride solution in tetrahydrofuran (0.5M, 100 mL, 50mmol). The reaction mixture is chilled to O0C and anhydrous ferric chloride (5.61g, 34.6mmol) was added in portions keeping the temperature below 1O0C. The resulting pale green solution was cooled to -350C and methyl magnesium chloride solution in tetrahydrofuran (3M, AImL, 141mmol) is added keeping the temperature below -3O0C. After the addition is complete the reaction mixture is cooled to -350C and 5α- pregnane-3, 20-dione (1Og, 31.65mmol) is added with stirring keeping the temperature below -250C. The reaction is allowed to warm to -2O0C and stirred at -180C to -220C for 3hrs. At this time there was 0.96% starting material by HPLC and 94.46% ganaxolone (Table 1, entry 6). The reaction is quenched by the slow addition of 225 ml of 3N HCl keeping the temperature below 250C. After the addition is complete the resulting suspension of ganaxolone is granulated overnight under nitrogen atmosphere. The reaction is filtered and the filter cake washed successively with 50 ml of 20% THF/3N HCl, 5OmL of 3N HCl, and twice with 50 ml of water. The filter cake is dried in a vacuum oven at 7O0C to afford 9.54 g (91% yield) of 99% pure ganaxolone 1 as a white solid.
References:
WO2011/19821,2011,A2 Location in patent:Page/Page column 12

566-65-4
163 suppliers
$50.00/1g

75-16-1
270 suppliers
$12.00/10ml

38398-32-2
75 suppliers
$28.00/1mg
![1-((2R,10S,13S,17S)-10,13-dimethylhexadecahydrospiro[cyclopenta[a]phenanthrene-3,2-oxiran]-17-yl)ethanone(WXG00316)](/CAS/20180703/GIF/148256-45-5.gif)
148256-45-5
2 suppliers
inquiry

38398-32-2
75 suppliers
$28.00/1mg

55571-86-3
1 suppliers
inquiry

38398-32-2
75 suppliers
$28.00/1mg

566-65-4
163 suppliers
$50.00/1g

38398-32-2
75 suppliers
$28.00/1mg