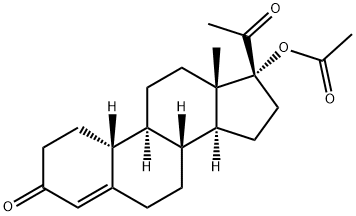
Gestonoronacetat synthesis
- Product Name:Gestonoronacetat
- CAS Number:31981-44-9
- Molecular formula:C22H30O4
- Molecular Weight:358.47
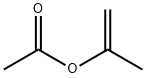
108-22-5
264 suppliers
$19.00/25mL
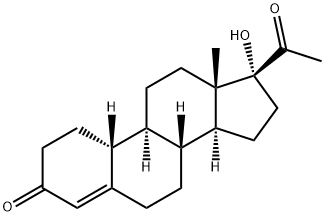
2137-18-0
81 suppliers
$87.00/25mg

31981-44-9
83 suppliers
inquiry
Yield:31981-44-9 82.36%
Reaction Conditions:
Stage #1:Isopropenyl acetate;17-alpha-Hydroxy-19-norprogesterone with toluene-4-sulfonic acid in dichloromethane;chloroform at 58 - 60; for 2 h;
Stage #2: with hydrogenchloride in dichloromethane;chloroform;water at 60;Solvent;Temperature;
Steps:
5 S5: esterification reaction
The compound 1W 5 prepared in S4 was added to the reaction flask, add 4.5 W 5 dichloromethane, 4.5 W5 trichloromethane stir well, adding 0.05 W 5 p-toluenesulfonic acid, heating up to 58 °C, adding 0.8 W 5 isopropenyl acetate, temperature control 64 °C reaction 2h, TLC monitoring to complete reaction; cooling to 60 , add 2.4 W 5 dilute hydrochloric acid hydrolysis 2 ~ 3h, TLC monitoring to complete reaction, static stratification, organic layer First with sodium bicarbonate solution extraction time, and then extracted with water once, all the water layers were combined and the aqueous layer was extracted with chloroform. The organic layers were combined, concentrated under reduced pressure and concentrated with methanol. Concentrated dry, add 0.6 W 5 methanol, 0.3 W 5 ethyl acetate 60 reflux 30min, cooling 0-10 °C insulation crystallization, filter, the cake was dried to give compound VI, i.e., 4-ene-17α-acetate-19- norpregna -3,20-dione, the total molar yield was 82.36%. Liquid content of 99.65% .
References:
Guangxi Wan De Pharmaceutical Co., Ltd.;Jiang Qingfeng;Yang Kun;Zhang Chengguo;Wei Wengui CN106866768, 2017, A Location in patent:Paragraph 0090; 0100-0101

2137-18-0
81 suppliers
$87.00/25mg

108-24-7
0 suppliers
$14.00/250ML

31981-44-9
83 suppliers
inquiry

734-32-7
220 suppliers
$300.00/1 g

31981-44-9
83 suppliers
inquiry
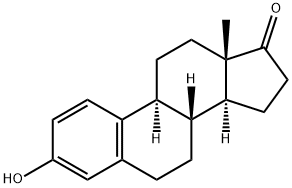
53-16-7
574 suppliers
$8.00/500mg

31981-44-9
83 suppliers
inquiry
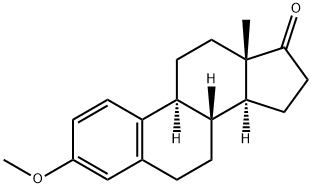
1624-62-0
178 suppliers
$35.00/1mg

31981-44-9
83 suppliers
inquiry