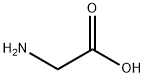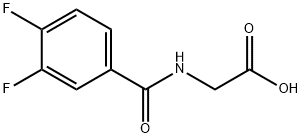
Glycine, N-(3,4-difluorobenzoyl)- synthesis
- Product Name:Glycine, N-(3,4-difluorobenzoyl)-
- CAS Number:315707-69-8
- Molecular formula:C9H7F2NO3
- Molecular Weight:215.15
Yield:315707-69-8 96%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran at 0 - 20;
Steps:
1
Step 1: Preparation of 2-(3,4-difluorobenzamido)acetic acid: Glycine (8.26 g, 110 mmol) was dissolved in 2M NaOH (125 ml, 250 mmol). The resulting colorless solution was cooled to 00C and 3,4-difluorobenzoyl chloride (12 mL, 95 mmol) in 60 mL of THF was added dropwise over 10 minutes. The reaction was stirred for 1 hour at 00C and allowed to warm to room temperature over 2 hours. The reaction was acidified to pH 1 with concentrated HCl while cooling in an ice bath. 100 mL of EtOAc was added and the mixture was poured into a separatory funnel and the layers separated. The aqueous layer was extracted with EtOAc (2 x 50 mL). The combined organic layers were washed with water and brine (50 mL each), dried with sodium sulfate, filtered and concentrated in vacuo to about 30 mL whereupon a solid began to crystallize. The mixture was allowed to stand overnight. The solid residue was concentrated on a rotary evaporator to remove the remaining EtOAc and then dried in a vacuum oven at 400C to provide the desired product, 2-(3,4-difluorobenzamido)acetic acid as an off-white solid (20.73 grams, 96%). LCMS (+ESI) m/z 216.2 [M+H]+. 1H-NMR (CDCl3) δ 12.70 (br s, IH), 9.00 (t, IH), 7.90 (m, IH), 7.75 (m, IH), 7.55 (m, IH), 3.90 (s, 2H). [00135]
References:
WO2010/68520,2010,A2 Location in patent:Page/Page column 26-27