
Glycine, N-(3-cyanophenyl)- synthesis
- Product Name:Glycine, N-(3-cyanophenyl)-
- CAS Number:91192-27-7
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
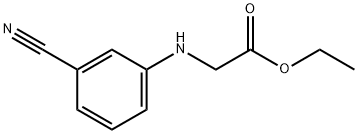
92316-76-2
1 suppliers
inquiry

91192-27-7
19 suppliers
inquiry
Yield:91192-27-7 97%
Reaction Conditions:
Stage #1: ethyl 2-(3-cyanophenylamino)acetatewith lithium hydroxide monohydrate;water in tetrahydrofuran; for 15 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; pH=3;
Steps:
153.B 1-(3-amidinophenyl)-5-[(2'-aminosulfonyl-[1,1']-biphen-4-yl)aminocarbonyl]-3-(methylthio)pyrazole, trifluoroacetic acid salt
Part B. Preparation of N-(3-cyanophenyl)glycine. To a solution of 17.00 g (83.2 mmol) of ethyl N-(3-cyanophenyl)glycine in 100 mL of THF under N2 was added 3.67 g (87.4 mmol) of lithium hydroxide monohydrate in 20 mL water. After 15 hours, the mixture was acidified with concentrated hydrochloric acid to pH 3 and a precipitate formed. The solid was collected, washed with 100 mL water and then dried in vacuo to give 14.15 g (97%) of the desired compound as a light yellow solid. 1H-NMR(CDCl3)δ: 7.28 (dt, 1H); 7.05 (dd, 1H): 6.83 (dd, 1H); 6.82 (d, 1H); 4.00 (s, 2H).
References:
EP946508,2009,B1 Location in patent:Page/Page column 70
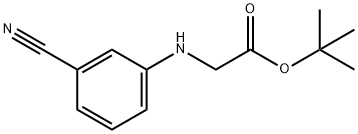
194162-74-8
0 suppliers
inquiry

91192-27-7
19 suppliers
inquiry
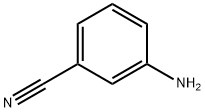
2237-30-1
271 suppliers
$13.00/25g

91192-27-7
19 suppliers
inquiry