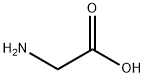
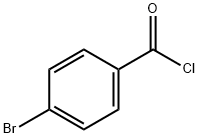
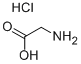
Glycine,N-(4-bromobenzoyl)- synthesis
- Product Name:Glycine,N-(4-bromobenzoyl)-
- CAS Number:18815-75-3
- Molecular formula:C9H8BrNO3
- Molecular Weight:258.07
Yield:18815-75-3 96.89%
Reaction Conditions:
with alkaline hydroxide at 0; for 0.5 h;
Steps:
1 2.1.1 Preparation of 4-Bromohippuric acid (1)
According to previously published procedure [32], a solution of glycine (8.25gm, 0.11Mol) and potassium hydroxide (0.2Mol, in 50mL) was cooled to 0°C then 4-Bromobenzoylchlororide (21.95gm, 0.1Mol) was added in two portions with shaking. Further, the reaction mixture was stirred for 30 min. After that, the solution was acidified with conc. HCl and the product was collected (25gm) and recrystallized from ethanol. Yield: 96.89%, M.P: found (155-157) °C, IR. (KBr) (νmax, Cm-1) 3296 (NH), 2550-3296 (acid OH), 1703 (acid C=O), 1635 (amide C=O).
References:
Samad, Mohammed Kareem;Hawaiz, Farouq Emam [Bioorganic Chemistry,2019,vol. 85,p. 431 - 444]

138119-50-3
1 suppliers
inquiry

18815-75-3
13 suppliers
inquiry
586-76-5
486 suppliers
$10.00/1g

18815-75-3
13 suppliers
inquiry

99208-44-3
9 suppliers
inquiry

18815-75-3
13 suppliers
inquiry