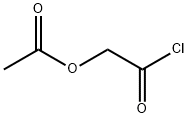
Glycolic Anhydride Diacetate synthesis
- Product Name:Glycolic Anhydride Diacetate
- CAS Number:25769-61-3
- Molecular formula:C8H10O7
- Molecular Weight:218.16
Yield:-
Reaction Conditions:
with pyridine at 20; for 4 h;
Steps:
2-(2-Chloroacetylaminopyridin-4-ylmethylthio)-N-(3,5-dimethylphenyl)pyridine-3-carboxamide (17)
General procedure: Acetoxyacetic acid (3.8 g, 32 mmol) was dissolved in pyridine (18 ml) at room temperature, acetoxyacetyl chloride (3.3 mL, 32 mmol) was added thereto, and the mixture was stirred for 4 hours at room temperature. In addition, 2-(2-aminopyridin-4-ylmethylthio)-N-(3,5-dimethylphenyl)pyridine-3-carboxamide compound 9e, 3.0 g, 8.1 mmol) was added thereto, and the mixture was stirred for 15 hours. Ethyl acetate (300 mL) was added to the reaction mixture, and the whole was washed with 1 N hydrochloric acid (100 mL) twice, saturated aqueous sodium hydrogen carbonate solution (100 mL) and brine (100 mL). The organic layer was dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure, and the resulting solid was filtered off with diethyl ether and ethyl acetate. Then the solid was dried under reduced pressure to give 3.3 g of the intermediate (2-(2-acetoxyacetylaminopyridin-4-ylmethylthio)-N-(3,5-dimethylphenyl)pyridine-3-carboxamide) as a pale yellow solid. The intermediate (3.3g, 7.1 mmol) was dissolved in the mixture of methanol (10 mL) and tetrahydrofuran (50 mL), and 1M aqueous sodium hydroxide solution (10mL) was added thereto. The mixture was stirred for 5 minutes at room temperature and diluted with ethyl acetate (200 mL), and then the whole was washed with brine (100 mL). The organic layer was dried over anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure. The resulting solid was filtered off with diethyl ether to give 2.4 g of the intermediate (2-(2-hydroxyacetylaminopyridin-4-ylmethylthio)-N-(3,5-dimethylphenyl)pyridine-3-carboxamide) as a pale yellow solid. (Yield 69% in 2steps) The intermediate (1.8 g, 4.2 mmol) was suspended in anhydrous dichloromethane (20 mL) under ice-cooling, and thionyl chloride (1.1 mL, 9.2 mmol) was added thereto. The mixture was stirred for 2 hours at room temperature, and the solvent was evaporated under reduced pressure. The resulting solid was filtered off with diethyl ether to give 1.6 g of the title compound as a pale yellow solid. (Yield 81%)
References:
Tajima, Hisashi;Honda, Takahiro;Kawashima, Kenji;Sasabuchi, Yoshimasa;Yamamoto, Minoru;Ban, Masakazu;Okamoto, Kazuyoshi;Inoue, Kenji;Inaba, Takaaki;Takeno, Yuriko;Tsuboi, Takashi;Tonouchi, Asaka;Aono, Hiroyuki [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 4,p. 1232 - 1235] Location in patent:supporting information; experimental part
13831-30-6
102 suppliers
$18.00/1g

25769-61-3
7 suppliers
inquiry