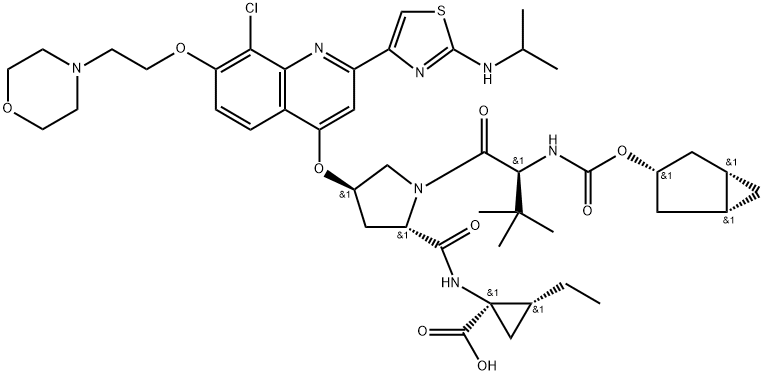
GS 9451 synthesis
- Product Name:GS 9451
- CAS Number:1098189-15-1
- Molecular formula:C45H60ClN7O9S
- Molecular Weight:910.53
![Cyclopropanecarboxyl?ic acid, N-?[[(1α,?3β,?5α)?-?bicyclo[3.1.0]?hex-?3-?yloxy]?carbonyl]?-?3-?methyl-?L-?valyl-?(4R)?-?4-?[[8-?chloro-?2-?[2-?[(1-?methylethyl)?amino]?-?4-?thiazolyl]?-?7-?[2-?(4-?morpholinyl)?ethoxy]?-?4-?quinolinyl]?oxy]?-?L-?prolyl-?1-?amino-?2-?ethyl-?, methyl ester, (1R,?2R)?-](/CAS/20211123/GIF/1098189-14-0.gif)
1098189-14-0
1 suppliers
inquiry

1098189-15-1
10 suppliers
inquiry
Yield:1098189-15-1 93%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran;methanol at 20; for 20 h;
Steps:
3 Example 3: Preparation of Compound 3
Example 3: Preparation of Compound 3 Compound 315 (12 g, 13 mmol) was dissolved in THF (200 ml), LiOH (11g, 260 mmol) in H20 (200 ml) was added, followed by MeOH (200 ml). The mixture was kept stirring at room temperature for 20 hours. Upon completion of the reaction, 4 N HCI in H20 was added to adjust pH to 7 at 0 °C. The mixture was extracted with EtOAc (2 x 400 ml). The combined organic layer was washed with brine, dried (Na2S04) and concentrated in vacuo to give compound 3 as a yellow solid (11 g, 93%). LC/MS = 911.52(M++1 ). 1H NMR (300MHz, CD3OD) 57.95 (d, 1H), 7.90 (s, 1H), 7.48 (s, 1H), 7.31 (d, 1H), 5.42 (s, 1H), 4.37 (dd, 1H), 4.20 (m, 2H), 3.83-3.56 (m, 7H), 3.50 (m, 2H), 3.39 (m, 2H), 2.45 (m, 1H), 2.27(m, 1H), 1.62 (m, 2H), 1.50 (m, 1H), 1.33 (m, 2H), 1.18 (m, 1H), 1.05 (m, 8H), 0.90 (m, 3H), 0.76 (m, 11H), 0.14-0.04 (m, 2H)
References:
GILEAD SCIENCES, INC.;RAY, Adrian S.;WATKINS, William J.;LINK, John O.;OLDACH, David W.;DELANEY, IV, William E. WO2013/40492, 2013, A2 Location in patent:Page/Page column 67

923289-36-5
70 suppliers
$16.00/100mg

1098189-15-1
10 suppliers
inquiry
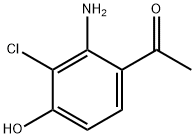
1098189-00-4
1 suppliers
inquiry

1098189-15-1
10 suppliers
inquiry
![Ethanone, 1-?[2-?amino-?3-?chloro-?4-?(2,?2-?dimethoxyethoxy)?phenyl]?-](/CAS/20210111/GIF/1098189-01-5.gif)
1098189-01-5
1 suppliers
inquiry

1098189-15-1
10 suppliers
inquiry
![4-?Quinolinol, 8-?chloro-?7-?(2,?2-?dimethoxyethoxy)?-?2-?[2-?[(1-?methylethyl)?amino]?-?4-?thiazolyl]?-](/CAS/20200611/GIF/1098189-06-0.gif)
1098189-06-0
1 suppliers
inquiry

1098189-15-1
10 suppliers
inquiry