Yield: 89%
Reaction Conditions:
with potassium hydroxide;water in 1,4-dioxane at 85 - 90; for 2 - 3 h;Product distribution / selectivity;
Steps:
116.D
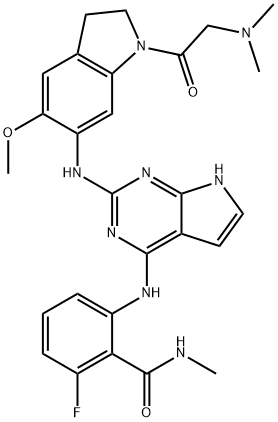
Step D/Example 116: 2-[(2-{[1-(λ/,λ/-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 H- indol-6-yl]amino}-1 /-/-pyrrolo[2,3-c/]pyrimidin-4-yl)amino]-6-fluoro-λ/-methylbenzamide; A light brown solution of 2-({2-{[1-(λ/,λ/-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 H- indol-6-yl]amino}-7-[(4-methylphenyl)sulfonyl]-7/-/-pyrrolo[2,3-c/]pyrimidin-4-yl}amino)- 6-fluoro-N-methylbenzamide (26 g, 37.9 mmol) prepared in multiple batches as described in Step C above, in dioxane (900 ml.) was treated with a 1 N aqueous KOH solution (379 ml_, 379 mmol). The resulting mixture was heated in a 90 0C bath for 3 hours. The resulting mixture was allowed to cool to room temperature, then was diluted with EtOAc (500 ml_). The organic layer was separated and sequentially washed with a 2N aqueous NaOH solution (2x200 ml.) and a saturated NaCI solution (300 ml_), then concentrated to a thick slurry. The resulting slurry was filtered and the solid washed with Et2O. The filtrate was concentrated to a slurry, filtered, and combined with the previous residue to obtain 2-[(2-{[1-(N,N-dimethylglycyl)-5- (methyloxy)-2,3-dihydro-1 H-indol-6-yl]amino}-1 H-pyrrolo[2,3-c/]pyrimidin-4-yl)amino]- 6-fluoro-N-methylbenzamide as a light brown solid. (16.9g, 84%, Yield). The remaining filtrate was concentrated and purified by flash silica gel chromatography using 1-10% MeOH (0.2% NH3) / CH2CI2 affording an additional 1 g (5%). 1H NMR (400 MHz, DMSO-de) δ ppm 2.28 (s, 6 H), 2.82 (d, J=4.40 Hz, 3 H), 3.08 - 3.15 (m, 2 H), 3.22 (s, 2 H), 3.79 (s, 3 H), 4.16 (t, J=8.25 Hz, 2 H), 6.27 (d, J=1.83 Hz, 1 H),6.80 - 6.89 (m, 1 H), 6.92 (d, J=2.20 Hz, 1 H), 6.93 (s, 1 H), 7.26 - 7.35 (m, 1 H), 7.38 (s, 1 H), 8.28 (s, 1 H), 8.36 (d, J=8.43 Hz, 1 H), 8.65 (s, 1 H), 10.10 (s, 1 H), 11.06 (s, 1 H). ESIMS (M+H) = 533.; Step D (alternative) / Example 116a (monohydrate preparation) 2-[(2-{[1-(λ/,λ/- dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 /-/-indol-6-yl]amino}-1 /-/-pyrrolo[2,3- c/]pyrimidin-4-yl)amino]-6-fluoro-λ/-methylbenzamide monohydrate.; A light brown solution of 2-({2-{[1-(λ/,λ/-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 H- indol-6-yl]amino}-7-[(4-methylphenyl)sulfonyl]-7H-pyrrolo[2,3-c/]pyrimidin-4-yl}amino)- 6-fluoro-N-methylbenzamide (19.1 kg, 27.8 mol), a method for preparation of which is described in Step C above, was suspended in dioxane (191 L, 10 volumes) and treated with a 15% aqueous KOH solution (76.4 L, 4 volumes). The mixture was heated to reflux (approximately 85 0C). Once the suspension became homogeneous, the reaction mixture was kept at reflux for 2 hours. In-process monitoring after 2h showed completion. The heat was discontinued and the suspension was concentrated to remove approximately 80 L of solvent. Water (141 L) was added and the suspension was stirred at ambient temperature for 1 h and filtered through centrifuge. The solid was washed with water till the filtrate ran clear to obtain 2-[(2- {[1-(N,N-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 H-indol-6-yl]amino}-1 H- pyrrolo[2,3-c/]pyrimidin-4-yl)amino]-6-fluoro-N-methylbenzamide monohydrate as a light brown solid (14.1 kg, 95% yield).NMR analysis confirmed 2-[(2-{[1-(N,N-dimethylglycyl)-5-(methyloxy)-2,3-dihydro-1 H- indol-6-yl]amino}-1 H-pyrrolo[2,3-c/]pyrimidin-4-yl)amino]-6-fluoro-N-methylbenzamide. Powder x-ray diffraction confirmed existence of the monohydrate, characterized by the pattern shown in Figure 1 , including the peaks of Table 1.
References:
SMITHKLINE BEECHAM CORPORATION WO2009/20990, 2009, A1 Location in patent:Page/Page column 360-362
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
