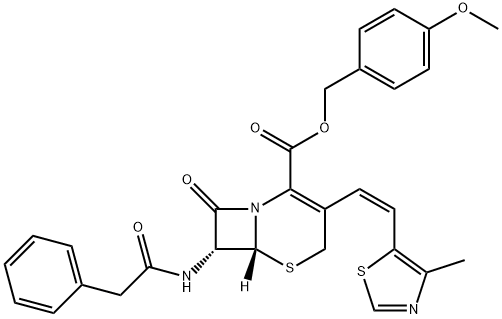
GTRE synthesis
- Product Name:GTRE
- CAS Number:138514-31-5
- Molecular formula:C29H27N3O5S2
- Molecular Weight:561.67
82294-70-0
284 suppliers
$6.00/1g

104146-10-3
260 suppliers
$5.00/5g

138514-31-5
3 suppliers
inquiry
Yield:138514-31-5 96.2%
Reaction Conditions:
Stage #1: (6R,7R)-3-Chloromethyl-8-oxo-7-phenylacetylamino-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid 4-methoxy-benzyl esterwith triphenylphosphine;sodium iodide in water;ethyl acetate at 30; for 1.5 h;Large scale;
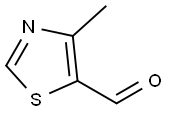
Stage #2: 4-methyl-1,3-thiazole-5-carbaldehyde in water;ethyl acetate at -25; pH=7;Large scale;Solvent;
Steps:
3 7-Phenylacetamido-3-[(Z)-2-(4-methyl-5-thiazolyl)ethenyl]-4-cephalosporanic acid p-methoxybenzyl ester
100 L of purified water, 100 L of ethyl acetate, 25 kg of GCLE, 8.2 kg of anhydrous sodium iodide were charged in a reaction kettle, Triphenylphosphine 14.5 kg, and reacted at 30 ° C for 1.5 hours. When the temperature was lowered to 0 ° C, the pH was adjusted to 7 by dropping 2% sodium hydroxide. The layers were separated, the methylene chloride layer was cooled to -25 ° C, 20 kg of 4-methyl-5-thiazolecarbaldehyde was added, and the reaction was allowed to proceed overnight. The reaction is complete, add 5% sodium bisulfite solution 150L, stirring for half an hour. The layers were separated, the ethyl acetate layer was concentrated to dryness in vacuo, 200 L of methanol was added, and the mixture was stirred at 0 ° C for 2 hours. The reaction mixture was centrifuged to give 27.7 kg of the title compound in 96.2% yield.
References:
CN103695522,2016,B Location in patent:Paragraph 0030-0032

104146-10-3
260 suppliers
$5.00/5g

138514-31-5
3 suppliers
inquiry

82294-70-0
284 suppliers
$6.00/1g
![Phosphonium, [[(6R,7R)-2-[[(4-methoxyphenyl)methoxy]carbonyl]-8-oxo-7-[(2-phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]triphenyl-, iodide (1:1)](/CAS/20211123/GIF/119636-62-3.gif)
119636-62-3
0 suppliers
inquiry

138514-31-5
3 suppliers
inquiry
![5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(1E)-2-(4-methyl-5-thiazolyl)ethenyl]-8-oxo-7-[(2-phenylacetyl)amino]-, (4-methoxyphenyl)methyl ester, (6R,7R)-](/CAS/20210305/GIF/104146-11-4.gif)
104146-11-4
1 suppliers
inquiry