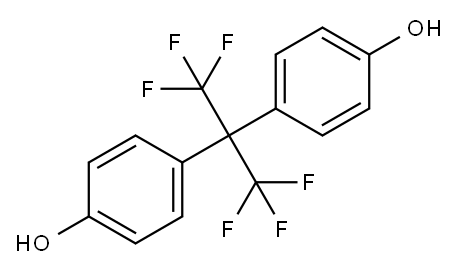
Hexafluorobisphenol A synthesis
- Product Name:Hexafluorobisphenol A
- CAS Number:1478-61-1
- Molecular formula:C15H10F6O2
- Molecular Weight:336.23
Yield:1478-61-1 97.3%
Reaction Conditions:
with TiClF3;SbF4Cl;hydrogen fluoride for 12 h;Autoclave;Reagent/catalyst;
Steps:
6
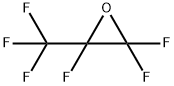
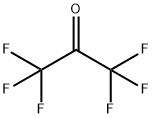
400 g of phenol was put into a 5-liter autoclave of a nickel alloy steel, and 1600 g of anhydrous hydrogen fluoride was charged through a pipe, 100 g of SbClF4And 100 g TiClF3, And then connected to the pipe of a cylinder of hexafluoropropylene oxide, 800 g of hexafluoropropylene oxide was added.After the inlet and outlet valves were closed, the stirring was started, heated to an internal temperature of 55 ° C, kept warm and stirred for 12 hours.After the reaction, the reactor was cooled to room temperature, and the reaction product was slowly introduced into 10 liters of cold water. The product was precipitated as a solid. The solid product was filtered off, washed with water, neutralized, dried and then recrystallized from ethanol to give a white crystalline solid. The melting point of 160 ° C, the product is bisphenol AF, weight 696 grams, the calculated yield of 97.3%.
References:
CN104370669,2016,B Location in patent:Paragraph 0039-0040