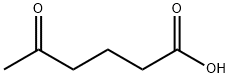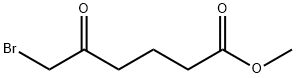
Hexanoic acid, 6-bromo-5-oxo-, methyl ester synthesis
- Product Name:Hexanoic acid, 6-bromo-5-oxo-, methyl ester
- CAS Number:68192-98-3
- Molecular formula:C7H11BrO3
- Molecular Weight:223.06
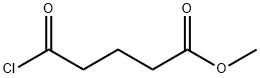
1501-26-4
145 suppliers
$8.00/1g

68192-98-3
1 suppliers
inquiry
Yield:68192-98-3 93%
Reaction Conditions:
Stage #1: methyl 4-(chlorocarbonyl)butyratewith diazomethane in diethyl ether;dichloromethane at 0; for 1.5 h;
Stage #2: with hydrogen bromide in diethyl ether;dichloromethane;water;
Steps:
4
6-bromo-5-oxo-hexanoic acid methyl ester. Methyl glutaryl chloride (2.5 mL, 18.23 mmol) was dissolved into anhydrous dichloromethane (10 mL) and added drop-wise to a 0° C. solution of CH2N2 (55.0 mmol generated from Diazald diazomethane precursor/KOH) in diethyl ether (150 mL). This solution was stirred at 0° C. for 1.5 h at which time the reaction was quenched via the drop-wise addition of 48% HBr (7.5 mL). The reaction mixture was diluted with dichloromethane (25 mL) and immediately washed with sat. NaHCO3 (3*25 mL) and brine (2*25 mL) before being dried (MgSO4), filtered and concentrated. The crude oil was purified via flash column chromatography (10-30% EtOAc/Hexanes) to obtain the title compound (3.76 g, 93%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 3.91 (s, 2H), 3.68 (s, 3H), 2.76 (t, 2H, J=7.2 Hz), 2.38 (t, 2H, J=7.2 Hz), 1.95 (quint, 2H, J=7.2 Hz); 13C NMR (75 MHz, CDCl3) δ 201.36, 173.41, 51.67, 38.74, 34.16, 32.87, 19.13; HRMS (ESI) calcd for C7H12O3Br (MH)+222.9964, found 222.9964.
References:
US2008/181923,2008,A1 Location in patent:Page/Page column 31

76700-84-0
0 suppliers
inquiry

68192-98-3
1 suppliers
inquiry
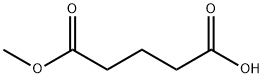
1501-27-5
191 suppliers
$19.00/5g

68192-98-3
1 suppliers
inquiry

116981-01-2
6 suppliers
$106.00/250mg

68192-98-3
1 suppliers
inquiry