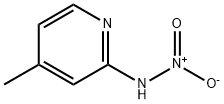
hydroxy-[(4-methylpyridin-2-yl)amino]-oxo-azanium synthesis
- Product Name:hydroxy-[(4-methylpyridin-2-yl)amino]-oxo-azanium
- CAS Number:33245-30-6
- Molecular formula:C6H7N3O2
- Molecular Weight:153.14
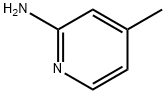
695-34-1
537 suppliers
$5.00/10g
![hydroxy-[(4-methylpyridin-2-yl)amino]-oxo-azanium](/CAS/GIF/33245-30-6.gif)
33245-30-6
29 suppliers
inquiry
Yield:33245-30-6 84%
Reaction Conditions:
Stage #1: 2-Amino-4-methylpyridinewith sulfuric acid at 0 - 5; for 1 h;
Stage #2: with nitric acid in water at -3 - 3; for 2.25 h;
Steps:
1.1
Example 1-Step 1; In a 500 mL 3-neck flask, equipped with a stirrer and an internal thermocouple probe (JKEM) was charged 116 mL of concentrated sulfuric acid (H2SO4) and allowed to cool between 0-5° C. with an ice bath. Then 25 g of the compound of formula 1 was charged, over a period of one hour, via an addition funnel. After one hour, 15.5 mL (1 (eq.)) of concentrated nitric acid (69-71%) was added dropwise maintaining the reaction mixture between -3° to 0° C. with external cooling (ice/methanol) over a period of 1.25 hours. The mixture was allowed to stir between 0-3° C. for one hour, after which HPLC analysis revealed complete conversion of the compound of formula 1 to the compound of formula 2. The solution was slowly quenched over 600 g of we ice in a 1 L beaker. The ice was allowed to melt and the resulting white slurry was stirred at room temperature for 10 min. The slurry was vacuum filtered, dried in vacuo (5 in Hg) at 50° C. (overnight) giving 84% recovered yield of the compound of formula 2. Analytical data: 1H-NMR (CD3OD) (δ, ppm): 2.50 (s, 3H), 7.20 (bd, 1H, J=6.6 Hz, Ar-H), 7.30 (bs, 1H, Ar-H), 8.03 (d, 1H, J=6.6 Hz, Ar-H): 13C-NMR (CD3OD) (δ, ppm): 21.00, 118.54, 119.91, 135.34, 144.00, 158.46: HRMS calcd for C6H7N3O2 153.05383 found (M+1) 154.06166.
References:
US2007/32657,2007,A1 Location in patent:Page/Page column 2
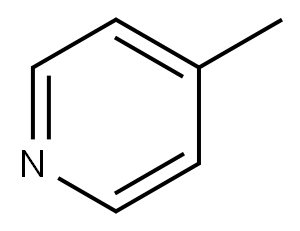
108-89-4
330 suppliers
$16.00/25mL
![hydroxy-[(4-methylpyridin-2-yl)amino]-oxo-azanium](/CAS/GIF/33245-30-6.gif)
33245-30-6
29 suppliers
inquiry