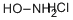
Hydroxylamine hydrochloride synthesis
- Product Name:Hydroxylamine hydrochloride
- CAS Number:5470-11-1
- Molecular formula:NH2OH·HCl
- Molecular Weight:69.49
100-64-1
270 suppliers
$5.00/25g
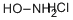
5470-11-1
750 suppliers
$10.00/5G
Yield:-
Reaction Conditions:
with hydrogenchloride in hexane;water at 40; under 2250.23 Torr; for 0.666667 h;
Steps:
6 Example 6
(1) cyclohexanone ammoximation reactionIn the presence of n-hexane, cyclohexanone (mass purity 96%), ammonia and hydrogen peroxide were added to the ammoximation reactor at molar ratio of 1: 0.93: 0.92 and TS-1 catalyst concentration of 3.5%A heterogeneous ammoximation reaction was carried out at 87 ° C, 0.4 MPa (G) and a stirring speed of 90 rpm,After 60 min reaction, the reaction mixture is obtained, and then the catalyst is separated to obtain a reaction liquid B;(2) cyclohexanone hydroxylamine oximation reactionThe reaction solution B obtained in step (1) and an appropriate amount of hydroxylamine are added to the hydroxylamine oximation reactor,Unconverted cyclohexanone reacted with hydroxylamine in reaction solution B at 30 , 0.2MPa (G) and stirring speed of 90 rpm,The reaction was 60 min after the reaction liquid C;The conversion of cyclohexanone is 100%, wherein the molar ratio of unconverted cyclohexanone to hydroxylamine in reaction solution B is 0.97: 1; (3) ExtractionThe reaction liquid C obtained in the step (2) is subjected to n-hexane extraction and separation,The organic phase E was extracted and the inorganic phase F was extracted,The organic phase E is extracted with cyclohexanone oxime n-hexane solution,Extract the inorganic phase F is slightly alkaline water containing n-hexane and ammonia,The volume ratio of extraction organic phase E to extraction inorganic phase F is 2.7: 1;(4) desolvent, cyclohexanone oxime refinedThe whole organic phase E obtained in step (3) is subjected to n-hexane removal and cyclohexanone oxime purification to obtain cyclohexanone oxime product G, and the n-hexane obtained after the removal is returned to the extraction section of step (3) for recycling;The extracted inorganic phase F obtained in the step (3) is stripped to recover n-hexane and then discharged as production waste water after environmental protection; (5) oxime hydrolysis and phase separationA portion of the cyclohexanone oxime product G obtained in the step (3) was dissolved in n-hexane and added to a suspension obtained by adding water to an oxime hydrolysis reactor charged with a cationic resin-type solid acid catalyst,The catalyst concentration was 6% (wt)The hydrolyzate J was obtained by reaction at 40 , 0.3MPa (G) and stirring speed of 90 rpm for 40 min. The hydrolyzate J was obtained by gravity sedimentation, and the hydrolyzed organic phase K and the hydrolyzed inorganic phase L were obtained.Hydrolysis of the organic phase K as a recycled material back to step (1) into the ammoximation reactor for ammoximation;A part of hydrolyzed inorganic phase L back to step (2) as a raw material instead of hydroxylamine into the hydroxylamine oximation reactor involved in the hydroxylamine oximation reaction;(6) hydroxylamine aqueous solution and hydroxylamine salt product preparationThe remaining portion of the hydrolyzed inorganic phase L obtained in step (5) was partially concentrated at 35 ° C and 7 kPa (a) to obtain a product hydroxylamine aqueous solution M having a concentration of 38% by weight. A portion thereof was neutralized with hydrochloric acid to obtain the product hydroxylamine hydrochloride.
References:
CN107089924,2017,A Location in patent:Paragraph 0015; 0030; 0031
7758-09-0
185 suppliers
$10.00/10g
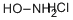
5470-11-1
750 suppliers
$10.00/5G
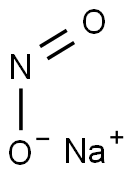
7632-00-0
858 suppliers
$14.00/25g
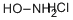
5470-11-1
750 suppliers
$10.00/5G

127-06-0
401 suppliers
$5.67/25g

51060-05-0
0 suppliers
inquiry
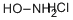
5470-11-1
750 suppliers
$10.00/5G
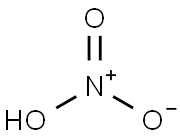
7697-37-2
8 suppliers
$13.00/25g
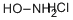
5470-11-1
750 suppliers
$10.00/5G