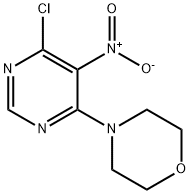
IFLAB-BB F2124-0140 synthesis
- Product Name:IFLAB-BB F2124-0140
- CAS Number:54660-14-9
- Molecular formula:C8H9ClN4O3
- Molecular Weight:244.64
Yield:54660-14-9 87%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0; for 0.5 h;
Steps:
4.1.16 4-(6-chloro-5-nitropyrimidin-4-yl)morpholine (12a)
To a mixture of 4,6-dichloro-5-nitropyrimidine (0.970g, 5.0mmol) and TEA (0.506g, 5.0mmol) in THF (20mL) at 0°C was added morpholine (0.436g, 5.0mmol). After stirring at 0°C for 30min, the resulting mixture was diluted with water (100mL) and extracted with EtOAc (50mL×3). The combined organic layers were washed with water (50mL×2) and brine (50mL), dried over anhydrous Na2SO4, filtered and concentrated. The residue was purified by flash column chromatography (silica gel, PE/EtOAc=4:1)to afford the product as a yellow solid (1.064g, 87% yield). 1H NMR (400MHz, DMSO-d6) δ 8.53 (s, 1H), 3.68 (t, J=4.8Hz, 4H), 3.54 (t, J=4.8Hz, 4H). MS (ESI+) m/z 245.0 [M+H]+.
References:
Sun, Yan;Fu, Rong;Lin, Songwen;Zhang, Jingbo;Ji, Ming;Zhang, Yan;Wu, Deyu;Zhang, Kehui;Tian, Hua;Zhang, Mingyi;Sheng, Li;Li, Yan;Jin, Jing;Chen, Xiaoguang;Xu, Heng [Bioorganic and Medicinal Chemistry,2021,vol. 29,art. no. 115890]

110-91-8
704 suppliers
$9.00/1g

67-56-1
774 suppliers
$9.00/25ml
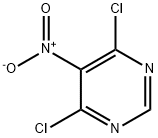
4316-93-2
286 suppliers
$12.00/5g

54660-14-9
17 suppliers
inquiry