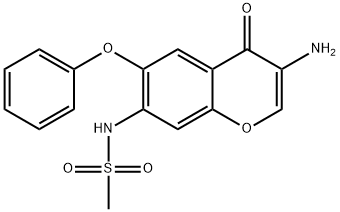
Iguratimod Impurity 1 synthesis
- Product Name:Iguratimod Impurity 1
- CAS Number:123663-48-9
- Molecular formula:C16H14N2O5S
- Molecular Weight:346.36
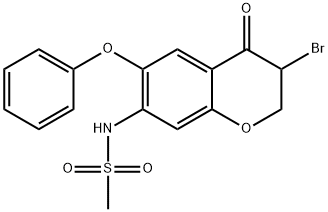
123664-34-6
1 suppliers
inquiry

123663-48-9
23 suppliers
inquiry
Yield:123663-48-9 82.1%
Reaction Conditions:
in water;ethyl acetate;N,N-dimethyl-formamide;
Steps:
40.2 Example 40
(2) In 280 ml of N,N-dimethylformamide was dissolved 40.1 g of 3-bromo-2,3-dihydro-7-methylsulfonylamino-6-phenoxy-4H-1-benzopyran-4-one. Thereto was added 13.9 g of sodium azide. The mixture was stirred for 1 hour at 70°-75° C. The reaction mixture was introduced into a mixed solvent consisting of 1.5 liters of water and 300 ml of ethyl acetate. The mixture was adjusted to pH 0.1 with conc. hydrochloric acid. The aqueous layer was separated, washed with 200 ml of ethyl acetate, adjusted to pH 4.0 with a 10% aqueous sodium hydroxide solution, and extracted with two 500-ml portions of ethyl acetate. The extracts (the organic layers) were combined, washed with water and a saturated aqueous sodium chloride solution in this order, and dried with anhydrous magnesium sulfate. The solvent was removed by distillation under reduced pressure. The crystal was recrystallized from ethanol to obtain 2.84 g (yield: 82.1%) of 3-amino-7-methylsulfonylamino-6-phenoxy-4H-1 -benzopyran-4-one having a melting point of 162°-163° C. IR (KBr) cm-1: 3440, 3330, 3180, 1600, 1580, 1550, 1480, 1465, 1330, 1205, 1150 NMR(d6 -DMSO)δ: 3.19 (3H, s), 5.50-7.00 (2H, br), 7.04-7.49 (5H, m), 7.35 (1H, s), 7.62 (1H, s), 7.94 (1H, s)
References:
US4954518,1990,A
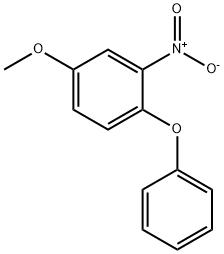
84594-95-6
59 suppliers
inquiry

123663-48-9
23 suppliers
inquiry
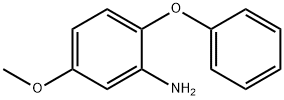
76838-72-7
68 suppliers
inquiry

123663-48-9
23 suppliers
inquiry
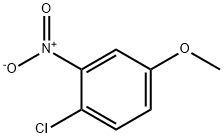
10298-80-3
335 suppliers
$10.00/10g

123663-48-9
23 suppliers
inquiry
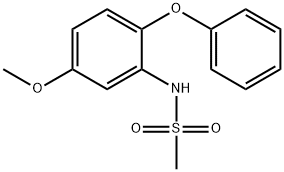
123664-84-6
102 suppliers
$19.00/100mg

123663-48-9
23 suppliers
inquiry