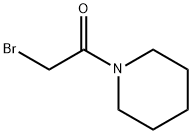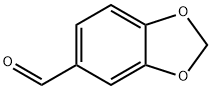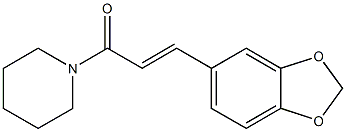
Ilepcimide synthesis
- Product Name:Ilepcimide
- CAS Number:82857-82-7
- Molecular formula:C15H17NO3
- Molecular Weight:259.3004
Yield:82857-82-7 87%
Reaction Conditions:
with polyethyleneimine supported triphenylphosphine in chloroform at 65;Inert atmosphere;Wittig Olefination;
Steps:
General Procedure for Wittig Reactions Using 8
General procedure: Polymer 8 (0.2 g, 0.4 mmol phosphine) was dissolved in CHCl3 (1 mL) in a 10-mL round-bottomed flask equipped with a magnetic stirrer and a reflux condenser, followed by 13a-b(0.3 mmol) and 16 (0.2 mmol). The mixture stirred at 65 C until the reaction was determined tobe complete by TLC or 1H NMR analysis. The reaction mixture was then cooled to rt and poured into a mixture of Et2O (10 mL) and hexane (30 mL) in a beaker. The flask was rinsed withadditional Et2O (10 mL), and the combined organic solution was allowed to stand for 10 minbefore it was filtered through a short pad of diatomaceous earth, using additional Et2O (2 x 10 mL)for rinsing. The filtrate was concentrated under reduced pressure to afford the desired product inan essentially pure state based on 1H NMR analysis
References:
Xia, Xuanshu;Toy, Patrick H. [Synlett,2015,vol. 26,# 12,art. no. ST-2015-W0209-L,p. 1737 - 1743] Location in patent:supporting information

96249-87-5
6 suppliers
inquiry

82857-82-7
5 suppliers
inquiry