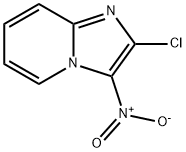
Imidazo[1,2-a]pyridine,2-chloro-3-nitro- synthesis
- Product Name:Imidazo[1,2-a]pyridine,2-chloro-3-nitro-
- CAS Number:4926-52-7
- Molecular formula:C7H4ClN3O2
- Molecular Weight:197.58
![IMidazo[1,2-a]pyridine,2-chloro-](/CAS/GIF/3999-05-1.gif)
3999-05-1
50 suppliers
$159.58/250mg
![Imidazo[1,2-a]pyridine,2-chloro-3-nitro-](/CAS/GIF/4926-52-7.gif)
4926-52-7
22 suppliers
$45.00/10mg
Yield:4926-52-7 91%
Reaction Conditions:
with sulfuric acid;HNO3 at 20; for 3 h;
Steps:
Synthesis methods of 2-chloro-3-nitro-H-imidazo[1,2-a]pyridine 5
A RBF immersed in ice bath containing 15 mL of H2SO4, 1 eq (1.5 g, 9.83 mmol) of 2-chloro-H-imidazo[1,2-a]pyridine 4 and 3.5 eq (1.6 mL, 34.40 mmol) of HNO3 were added. The reaction mixture was stirred at room temperature for 3 h and followed by TLC analysis. Reaction mixture was extracted with DCM and the organic layer was dried over Na2SO4. The organic phase was evaporated under vacuo, dried and without any further purification to give 1.76 g (91%) compound 5 as yellow crystals. M.p: 166°-168°C, 1H NMR (400 MHz, Acetone-d6) δ (ppm) 9.42 (dt, J = 7.0, 1.1 Hz, 1H; HAr), 7.92 - 7.79 (m, 2H; HAr), 7.52 (td, J = 7.0, 1.5 Hz, 1H; HAr). 13C NMR (100 MHz, Acetone-d6) δ (ppm) 132.08, 117.33. HRMS (ESI): Calc for C7H5ClN2O2 [M+H+] = 198.8974 Found = 198.8977
References:
Ablo, Evrard;Coulibali, Siomenan;Touré, Daouda;Coulibaly, Souleymane;Kablan, Ahmont Landry Claude;Konan Kouadio, Fernique;Sissouma, Drissa;Guessend Kouadio, Nathalie;Ané, Adjou [Synthetic Communications,2022,vol. 52,# 3,p. 462 - 469] Location in patent:supporting information
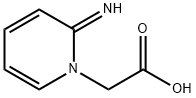
126202-06-0
23 suppliers
$165.00/2g
![Imidazo[1,2-a]pyridine,2-chloro-3-nitro-](/CAS/GIF/4926-52-7.gif)
4926-52-7
22 suppliers
$45.00/10mg
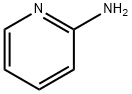
504-29-0
540 suppliers
$5.00/5G
![Imidazo[1,2-a]pyridine,2-chloro-3-nitro-](/CAS/GIF/4926-52-7.gif)
4926-52-7
22 suppliers
$45.00/10mg