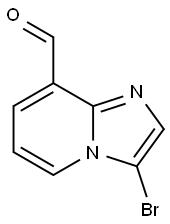
IMidazo[1,2-a]pyridine-8-carboxaldehyde, 3-broMo- synthesis
- Product Name:IMidazo[1,2-a]pyridine-8-carboxaldehyde, 3-broMo-
- CAS Number:1033434-54-6
- Molecular formula:C8H5BrN2O
- Molecular Weight:225.04
![Imidazo[1,2-a]pyridine-8-carboxaldehyde (9CI)](/CAS/GIF/136117-74-3.gif)
136117-74-3
63 suppliers
$43.00/100mg
![IMidazo[1,2-a]pyridine-8-carboxaldehyde, 3-broMo-](/CAS/GIF/1033434-54-6.gif)
1033434-54-6
27 suppliers
$45.00/10mg
Yield: 35%
Reaction Conditions:
with bromine in acetic acid at 20;
Steps:
31 3-Bromoimidazo[1,2-a]pyridine-8-carbaldehyde (4k)
Bromination of aldehydes 4a, bTo a solution of the appropriate aldehyde (1.39 mmol) in AcOH (6 mL) was added dropwise a solution of bromine (266 mg, 1.66 mmol) in AcOH (3 mL). The solution was stirred at room temperature for 3.5 h. The solution was diluted with water (6 mL), cooled to 0° C. and made basic by the addition of aqueous saturated Na2CO3 solution (30 mL) over 20 min. The solution was extracted with CH2Cl2 and the combinated extracts were dried (MgSO4), filtered and evaporated under reduced pressure to give bromo compound; Example 31 3-Bromoimidazo[1,2-a]pyridine-8-carbaldehyde (4k) From 4a (yield: 35%); mp 104-106° C.; IR (KBr) 1694, 1186 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.09 (t, 1H J=7 Hz), 7.74 (s, 1H), 7.86 (d, 1H, J=7 Hz), 8.32 (d, 1H, J=7 Hz), 10.66 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 96.4, 112.9, 124.2, 127.1, 128.5, 134.6, 144.2, 188.2; MS m/z 226 (M++2, 45), 224 (M+, 46), 198 (99), 196 (100), 117 (76), 90 (54), 63 (30).
References:
Universite D'Auvergne Clermont 1;Universite Francois Rabelais Tours;Katholieke Universiteit Leuven US2010/93781, 2010, A1 Location in patent:Page/Page column 22