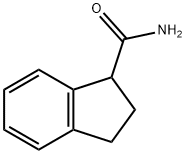
indan-1-carboxamide synthesis
- Product Name:indan-1-carboxamide
- CAS Number:33695-57-7
- Molecular formula:C10H11NO
- Molecular Weight:161.2
![1H-Indene-1-carboxamide, 2,3-dihydro-N-[(1,1,2,2,3,3,4,4,4-nonafluorobutyl)sulfonyl]-](/CAS/20210305/GIF/1440968-36-4.gif)
1440968-36-4
0 suppliers
inquiry

33695-57-7
16 suppliers
$265.00/100mg
Yield:33695-57-7 96%
Reaction Conditions:
with samarium diiodide in tetrahydrofuran at 23; for 0.333333 h;Inert atmosphere;
Steps:
4.7 Reductive desulfonylation of N-acyl sulfonamide 6g to afford carboxamide 12
Adapted from Ankner and Hilmersson.15 In a nitrogen-filled drybox, a flame-dried 10-mL round-bottomed glass flask fitted with a rubber septum and Teflon-coated magnetic stir bar was charged with a solution of samarium diiodide in tetrahydrofuran (0.1M, 3.38mL, 338μmol, 6.00equiv). To this solution was added a solution of N-acyl sulfonamide 6g (25.0mg, 56.4μmol, 1equiv) in tetrahydrofuran (300μL). The resulting mixture was stirred for 30min at 23°C. The reaction mixture was then removed from the drybox, and 1mL of saturated aqueous sodium bicarbonate solution was added with stirring. The resulting mixture was then concentrated to dryness under reduced pressure, was suspended in 5mL of methanol, and was filtered over a Celite pad, rinsing the reaction vessel and Celite pad with additional methanol (2×5mL). This filtrate was concentrated to dryness, and the residue obtained was purified by flash-column chromatography (eluting with ethyl acetate) to afford indan-1-carboxamide (12, 9.0mg, 96%, white solid). Rf=0.70 (15% methanol-ethyl acetate; UV, PMA). 1H NMR (500MHz, (CD3)2SO) δ 7.56 (br s, 1H), 7.27-7.26 (m, 1H), 7.23-7.21 (m, 1H), 7.16-7.14 (m, 2H), 6.96 (br s, 1H), 3.84 (app t, J=7.6Hz, 1H), 2.98 (app ddd, J=14.8, 8.8, 5.3Hz, 1H), 2.82 (app dt, J=15.8, 7.8Hz, 1H), 2.26-2.11 (m, 2H). 13C NMR (125MHz, (CD3)2SO), δ 174.85 (C), 143.9 (C), 142.9 (C), 126.8 (CH), 126.0 (CH), 124.3 (CH), 124.0 (CH), 50.4 (CH), 31.6 (CH2), 28.47 (CH2). FTIR (thin film), cm-1: 3357.5 (s), 3174.5 (s), 2159.9 (s), 2022.3 (s), 1646.9 (s), 1425.9 (s). HRMS-CI (m/z): [M+Na]+ calcd for C10H11NO, 184.0733; found 184.0732.
References:
Mitcheltree, Matthew J.;Konst, Zef A.;Herzon, Seth B. [Tetrahedron,2013,vol. 69,# 27-28,p. 5634 - 5639]
19156-10-6
0 suppliers
inquiry

33695-57-7
16 suppliers
$265.00/100mg
29427-69-8
104 suppliers
$25.00/1g

33695-57-7
16 suppliers
$265.00/100mg
14381-42-1
94 suppliers
$46.00/1g

33695-57-7
16 suppliers
$265.00/100mg
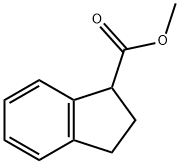
26452-96-0
9 suppliers
inquiry

33695-57-7
16 suppliers
$265.00/100mg