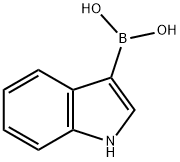
Indole-6-boronic acid synthesis
- Product Name:Indole-6-boronic acid
- CAS Number:147621-18-9
- Molecular formula:C8H8BNO2
- Molecular Weight:160.97
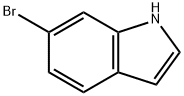
52415-29-9
414 suppliers
$9.00/1g

688-74-4
323 suppliers
$10.40/25mL
594-19-4
185 suppliers
$27.50/50ml

147621-18-9
132 suppliers
$22.00/100mg
Yield: 68%
Reaction Conditions:
with hydrogenchloride in tetrahydrofuran;methanol;dichloromethane;pentane
Steps:
474.A 1H-indol-6-ylboronic acid
EXAMPLE 474A 1H-indol-6-ylboronic acid A solution of 6-Bromo-1H-indole (1.5 g, 7.65 mmol) in THF (10 mL) was added dropwise to a suspension of potassium hydride (0.31 g, 7.65 mmol) in THF at 0° C. After fifteen minutes at 0° C., the solution was cooled to -78° C. and a t-butyl lithium solution (1.7 M in pentane, 9.0 mL, 15.3 mmol) was added dropwise via syringe while maintaining a temperature below -55° C. After 15 minutes, the solution was cooled to -78° C. and treated with a tributyl borate (4.14 mL, 15.3 mmol). The solution was stirred at -78° C. for 2 hours and then allowed to warm to -10° C. The solution was the added 75 mL of 1 M HCl, warmed to room temperature and separated. The aqueous phase was extracted with diethyl ether (3*75 mL) and the combined organics were extracted with 1 M NaOH (4*40 mL). The aqueous layers were combined, adjusted to pH 2 with 6 M HCl and extracted with diethyl ether (4*50 mL). The combined organic layers were washed with brine (20 mL), dried (MgSO4), filtred and concentrated. The concentrate was purified by flash chromatography on silica gel using 3.5-5% methanol/dichloromethane to give 838 mg (68% yield) of the desired product. MS (ESI(+)) m/e 161 (M+H)+.
References:
Betschmann, Patrick;Burchat, Andrew F.;Calderwood, David J.;Curtin, Michael L.;Davidsen, Steven K.;Davis, Heather M.;Frey, Robin R.;Heyman, Howard R.;Hirst, Gavin C.;Hrnciar, Peter;Michaelides, Michael R.;Muckey, Melanie A.;Rafferty, Paul;Wada, Carol K. US2005/26944, 2005, A1

52415-29-9
414 suppliers
$9.00/1g

147621-18-9
132 suppliers
$22.00/100mg

52415-29-9
414 suppliers
$9.00/1g

688-74-4
323 suppliers
$10.40/25mL

147621-18-9
132 suppliers
$22.00/100mg

52415-29-9
414 suppliers
$9.00/1g
13675-18-8
156 suppliers
$6.00/1g

147621-18-9
132 suppliers
$22.00/100mg
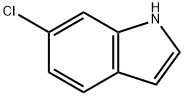
17422-33-2
312 suppliers
$10.00/1g

147621-18-9
132 suppliers
$22.00/100mg