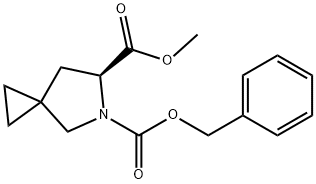
5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?- synthesis
- Product Name:5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?-
- CAS Number:1256388-46-1
- Molecular formula:C16H19NO4
- Molecular Weight:289.33

200184-60-7
22 suppliers
$130.00/250MG

75-11-6
344 suppliers
$10.00/5g
![5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?-](/CAS/20210111/GIF/1256388-46-1.gif)
1256388-46-1
7 suppliers
inquiry
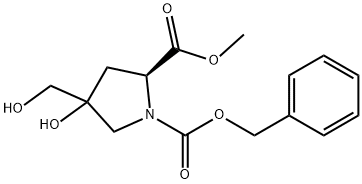
1352390-10-3
0 suppliers
inquiry
Yield:1256388-46-1 65%
Reaction Conditions:
Stage #1: diiodomethanewith diethylzinc;trifluoroacetic acid in hexane;dichloromethane at 0; for 0.4 h;Inert atmosphere;
Stage #2: (S)-N-benzyloxycarbonyl-4-methylenepyrrolidine-2-carboxylic acid methyl ester in hexane;dichloromethane at 0 - 20; for 110 h;Inert atmosphere;
Stage #3: with osmium(VIII) oxide;water;4-methylmorpholine N-oxide in tetrahydrofuran;acetone at 20; for 7 h;
Steps:
6.b; 6.c
b. Preparation of a mixture of Compounds 603 and 604. An oven-dried 3-neck round bottom flask was equipped with a nitrogen inlet adaptor and a 250 mL addition funnel. The third neck was sealed with a septum. The flask was charged with a stir bar, dichlorormethane (120 mL) and diethyl zinc (1.0 M in hexane, 1 18 mL, 118 mmol) then cooled to 0 °C in an ice bath. The addition funned was charged with dichloromethane (40 mL) and trifluoroacetic acid (9.1 mL, 118 mmol). After the diethyl zinc solution had cooled to 0 °C (about 25 minutes), the trifluoroacetic acid solution was added dropwise over 20 min to the stirred reaction mixture. After stirring for another 20 min at 0 °C, diiodomethane (9.5 mL, 1 18 mmol) was added slowly over 4 minutes. After another 20 min, 4- methylene-pyrrolidine-1 ,2-dicarboxylic acid 1 -benzyl ester 2-methyl ester 602 (8.10 g, 29.4 mmol) was added in 30 mL dichloromethane by cannula. The flask containing 4-methylene- pyrrolidine-1 ,2-dicarboxylic acid 1 -benzyl ester 2-methyl ester was then rinsed with another 10 mL dichloromethane and this solution was also transferred to the reaction mixture by cannula. The reaction mixture was allowed to warm to RT and stirred for 110 h (about 5 days) after which the reagents were quenched with saturated aqueous ammonium chloride (~150 mL). The contents of the flask were slowly poured into a 2 L sep funnel containing saturated aqueous sodium bicarbonate (800 mL). The aqueous phase was extracted three times with 300 mL ethyl acetate. The combined organics were dried over magnesium sulfate and concentrated to provide a mixture of Compounds 603 and 604. c. Preparation of a Compound 603. The crude material from sub-part b was dissolved in 3:1 :1 THF/water/acetone (165 mL) then treated with /V-methylmorpholine-/V-oxide (3.45 g, 29.4 mmol) and osmium tetroxide (4 wt% in water, 5 mL, 0.818 mmol). After stirring at RT for 7 h, the reagents were quenched with 1 M aqueous sodium thiosulfate (~100 mL). The contents of the flask were then poured into a 1 L sep funnel containing water (~300 mL). The aqueous phase was extracted three times with 300 mL dichloromethane. The combined organics were dried over magnesium sulfate and concentrated. The crude residue was purified by silica column chromatography (5% to 45% EtOAc hexane) to provide 5-aza-spiro[2.4]heptane-5,6-dicarboxylic acid 5-benzyl ester 6- methyl ester 603 as a clear oil (5.54g, 19.15 mmol, 65%) as a clear oil. 1H NMR (CDCI3) δ 7.36-7.29 (m, 5H), 5.21-5.04 (m, 2H), 4.56-4.47 (m, 1 H), 3.75 (s, 1.5H), 3.60 (m, 1.5H), 03.51- 3.37 (m, 2H), 2.32-2.25 (m, 1 H), 1.87-1.80 (m, 1 H), 0.64-0.51 (m, 4H).
References:
WO2013/40492,2013,A2 Location in patent:Page/Page column 86-87

200184-60-7
22 suppliers
$130.00/250MG

75-11-6
344 suppliers
$10.00/5g
![5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?-](/CAS/20210111/GIF/1256388-46-1.gif)
1256388-46-1
7 suppliers
inquiry

200184-60-7
22 suppliers
$130.00/250MG
![5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?-](/CAS/20210111/GIF/1256388-46-1.gif)
1256388-46-1
7 suppliers
inquiry

1352390-10-3
0 suppliers
inquiry

501-53-1
393 suppliers
$10.00/1g
![5-?Azaspiro[2.4]?heptane-?5,?6-?dicarboxylic acid, 6-?methyl 5-?(phenylmethyl) ester, (6S)?-](/CAS/20210111/GIF/1256388-46-1.gif)
1256388-46-1
7 suppliers
inquiry