ipalbidine synthesis
- Product Name:ipalbidine
- CAS Number:26294-41-7
- Molecular formula:C15H19NO
- Molecular Weight:229.32
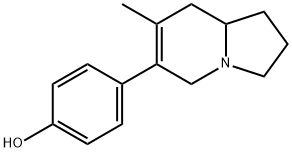
111751-72-5
0 suppliers
inquiry

26294-41-7
6 suppliers
inquiry
Yield:-
Steps:
Multi-step reaction with 9 steps
1: 1.) K(t-BuO) / 1.) 8percent aq. DMSO, 2.) aq. DMSO, 3h, 20 deg C
2: 74 percent / H2 / 5percent Pd/c / ethanol / 21 h / Ambient temperature
3: 86 percent / N-methylmorpholine / CH2Cl2 / 1 h, 0 deg C, then 30 min, room t.
4: N-methylmorpholine / diethyl ether / 40 min, 0 deg C, then 50 min, room t.
5: diethyl ether / 0 deg C, then 16 h, room t.
6: 82 percent / Rh(OAc)2 / CH2Cl2 / 1.5 h / Ambient temperature
7: 7 percent / H2 / Rh/act. Al2O3 / ethanol / 5 h / 50 °C / 3102.9 Torr
8: 1.) THF, 24 h, room t., 2.) THF
9: 48percent aq. HBr / 1 h / 80 °C
References:
Jefford, Charles W.;Kubota, Tadatoshi;Zaslona, Alexander [Helvetica Chimica Acta,1986,vol. 69,p. 2048 - 2061]
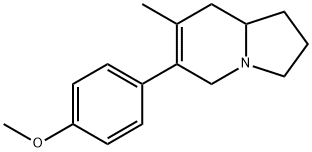
29079-30-9
0 suppliers
inquiry

26294-41-7
6 suppliers
inquiry
![7-Indolizinol, 6-[4-(acetyloxy)phenyl]octahydro-7-methyl-, 7-acetate](/CAS/20210305/GIF/111752-02-4.gif)
111752-02-4
0 suppliers
inquiry

26294-41-7
6 suppliers
inquiry