
ISIBNKKIJOEHIW-UHFFFAOYSA-N synthesis
- Product Name:ISIBNKKIJOEHIW-UHFFFAOYSA-N
- CAS Number:849210-10-2
- Molecular formula:C13H10BrNO3
- Molecular Weight:308.13
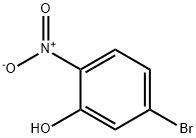
27684-84-0
156 suppliers
$9.00/1g

100-39-0
425 suppliers
$10.00/10g

849210-10-2
10 suppliers
inquiry
Yield:849210-10-2 97%
Reaction Conditions:
with potassium carbonate in acetone; for 5 h;Reflux;
Steps:
83.B
A mixture of 5-bromo-2-nitrophenol (10.4 g, 47.7 mmol), benzyl bromide (9.8 g, 57.2 mmol) and potassium carbonate (9.9 g, 71.5 mmol) was taken up in acetone (50 mL) and heated to reflux for 5 h. After cooling to ambient temperature, the reaction mixture was concentrated in vacuo, diluted with water and extracted with ethyl acetate. The organic layer was washed with brine, dried over sodium sulfate and concentrated in vacuo. The crude material was purified by flash column chromatography (9: 1 hexanes/ethyl acetate) to give 2-(benzyloxy)-4-bromo-l -nitrobenzene (14.19 g, 97%) as a light-yellow solid: 1H NMR (CDC13, 300 MHz) δ 7.76 (d, J= 8.4 Hz, 1H), 7.32-7.48 (m, 5H), 7.30 (d, J= 1.8 Hz, 1H), 7.19 (dd, J= 8.7, 1.8 Hz, 1H), 5.23 (s, 2H).
References:
WO2011/44134,2011,A1 Location in patent:Page/Page column 107