
isocyanato(methanesulfonyl)methane synthesis
- Product Name:isocyanato(methanesulfonyl)methane
- CAS Number:1161826-12-5
- Molecular formula:C3H5NO3S
- Molecular Weight:135.14
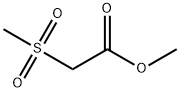
62020-09-1
86 suppliers
$19.00/5g

1161826-12-5
8 suppliers
inquiry
Yield:1161826-12-5 51%
Reaction Conditions:
Stage #1: Methyl α-(methylsulfonyl)acetatewith hydrazine in ethanol; for 1.5 h;Heating / reflux;
Stage #2: with hydrogenchloride;sodium nitrite in chloroform;water at 0; for 0.0833333 h;
Steps:
52
Synthesis 52S-Methanesulfonylmethyl^-oxo-S^-dihydro-imidazoIδ.i-dlti ^.S.Sltetrazine-δ-carboxylic acid amide (UU-002);
References:
WO2009/77741,2009,A2 Location in patent:Page/Page column 102-103