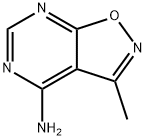
Isoxazolo[5,4-d]pyrimidin-4-amine, 3-methyl- (9CI) synthesis
- Product Name:Isoxazolo[5,4-d]pyrimidin-4-amine, 3-methyl- (9CI)
- CAS Number:89799-07-5
- Molecular formula:C6H6N4O
- Molecular Weight:150.14
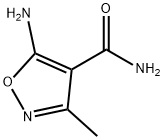
35261-06-4
25 suppliers
$64.00/250mg
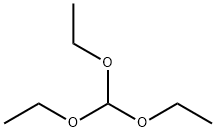
122-51-0
485 suppliers
$10.00/5ml
![Isoxazolo[5,4-d]pyrimidin-4-amine, 3-methyl- (9CI)](/CAS/GIF/89799-07-5.gif)
89799-07-5
19 suppliers
$45.00/100mg
Yield:89799-07-5 34%
Reaction Conditions:
Stage #1: 5-amino-3-methyl-1,2-oxazole-4-carboxamide;orthoformic acid triethyl ester in acetic anhydride at 20 - 160; for 3 h;Heating / reflux;
Stage #2: with ammonia in water;
Stage #3: with acetic acid in water;
Steps:
3.a
To an oven-dried one- neck round bottomed flask charged with a magnetic stir bar at rt under argon was added 5-amino-3-methylisoxazole-4-carboxamide (1.0 g, 7.1 mmol), acetic anhydride (7 mL) and triethylorthoformate (7 mL) and the mixture was refluxed for 3 h (oil bath at 160C). The resulting light orange solution was cooled to rt, evaporated to dryness and the residue was dissolved in minimum amount of concentrated ammoniurn hydroxide. The solution was acidified with glacial acetic acid and the mixture was cooled overnight in the refrigerator to obtain the title compound as an off-white solid (0.39 g, 34%).1H NMR (DMSO-d6) 8.36 (s, IH), 3.18 (s, IH), 2.48 (s, 3H).
References:
WO2008/57402,2008,A2 Location in patent:Page/Page column 50
5417-82-3
116 suppliers
$9.00/1g
![Isoxazolo[5,4-d]pyrimidin-4-amine, 3-methyl- (9CI)](/CAS/GIF/89799-07-5.gif)
89799-07-5
19 suppliers
$45.00/100mg

35261-01-9
62 suppliers
inquiry

122-51-0
485 suppliers
$10.00/5ml
![Isoxazolo[5,4-d]pyrimidin-4-amine, 3-methyl- (9CI)](/CAS/GIF/89799-07-5.gif)
89799-07-5
19 suppliers
$45.00/100mg