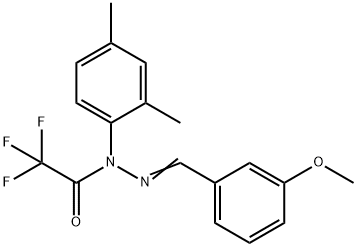
J 147 synthesis
- Product Name:J 147
- CAS Number:1146963-51-0
- Molecular formula:C18H17F3N2O2
- Molecular Weight:350.33
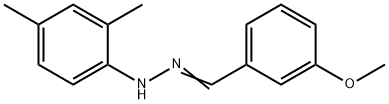
391646-35-8
0 suppliers
inquiry
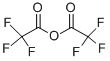
407-25-0
436 suppliers
$9.00/1g

1146963-51-0
164 suppliers
$6.00/5mg
Yield:1146963-51-0 83%
Reaction Conditions:
with triethylamine in dichloromethane at 0;Inert atmosphere;
Steps:
(c). N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N′-(3-methoxybenzylidene)acetohyrazide (J147, 2).
To a stirred solution of 1 (500 mg, 1.97 mmol) and Et3N (0.33 mL, 2.36 mmol) in CH2Cl2 (5 mL) was added trifluoroacetic anhydride (0.33 mL, 2.36 mmol) dropwise at 0 °C under nitrogen atmosphere. The reaction mixture was stirred at 0 °C for 1.5 h. After the mixture was concentrated, the residue was purified by column chromatography with acetone/hexanes (1:10) to afford compound 2 (571 mg, 83%) as a pale yellow oil. 1H NMR (CDCl3): δ 7.26-7.23 (m, 4H), 7.19 (d, J = 8.0 Hz, 1H), 7.12 (d, J = 7.5 Hz, 1H), 7.03 (d, J = 8.0 Hz, 1H), 6.92 (dd, J = 8.5, 2.0 Hz, 1H), 3.79 (s, 3H), 2.39 (s, 3H), 2.08 (s, 3H). HRMS (ESI-TOF, m/z): calcd for C18H18N2O2F3 ([M+H]+) 351.1320; found 351.1311.
References:
Wang, Min;Gao, Mingzhang;Zheng, Qi-Huang [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 2,p. 524 - 527]
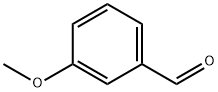
591-31-1
414 suppliers
$10.00/5g

1146963-51-0
164 suppliers
$6.00/5mg