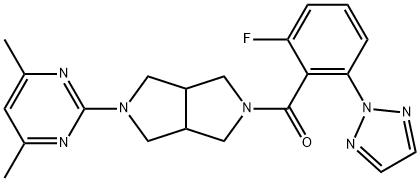
JNJ-42847922 synthesis
- Product Name:JNJ-42847922
- CAS Number:1293281-49-8
- Molecular formula:C21H22FN7O
- Molecular Weight:407.44
1186050-58-7
45 suppliers
$101.00/250mg
![Pyrrolo[3,4-c]pyrrole, 2-(4,6-dimethyl-2-pyrimidinyl)octahydro-](/CAS/20200611/GIF/1293284-71-5.gif)
1293284-71-5
5 suppliers
inquiry

1293281-49-8
42 suppliers
inquiry
Yield: 74%
Reaction Conditions:
with thionyl chloride;sodium carbonate;sodium hydroxide in water;toluene at 0 - 50; for 4.5 h;Inert atmosphere;
Steps:
107.A
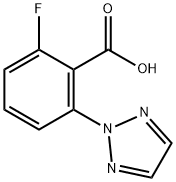
To a 3-necked, 3 L, round- bottomed flask equipped with a nitrogen line, temperature probe, heating mantle, reflux condenser, mechanical stirrer, and 1 N aq. NaOH scrubber were added 2-fluoro-6-[1 ,2,3]triazol-2-yl-benzoic acid (Intermediate 12, 120.98 g, 75 wt%, 90.74 g actual, 438 mmol) and toluene (1 L). The mixture was warmed to 50 oC for 1 h with stirring. The mixture was then cooled to 25 oC and thionyl chloride (47.9 mL, 657 mmol) was added. The mixture was warmed back to 50 oC and held for 1 h. During this time, in a separate 5 L jacketed reactor equipped with a mechanical stirrer and temperature probe were added toluene (600 mL), aqueous sodium carbonate (185.7 g, 1 .75 mol in 1 .6 L water), and 2- (4,6-dimethyl-pyrimidin-2-yl)-octahydro-pyrrolo[3,4-c]pyrrole*HOAc(Intermediate 23, 122 g, 438 mmol). This biphasic mixture was cooled to 0 oC. After cooling to 0 oC, the original slurry was poured through a filter and over the stirring biphasic mixture of amine and aqueous sodium carbonate. The mixture was allowed to warm to room temperature. After 2 h, additional 2-(4,6- dimethyl-pyrimidin-2-yl)-octahydro-pyrrolo[3,4-c]pyrrole*HOAc (4 g, 14 mmol) was added and the mixture was stirred for 30 additional minutes. At the end of this period, the layers were separated and 100 mL of methanol were added to the organic layer. The organic layer was dried over MgSO , filtered, and concentrated to a white solid. This solid was taken up in ethanol (1 .4 L) and warmed to 77 oC. The mixture was then cooled to 55 oC and seeded with previously crystallized material. (Note: The seeds were generated from slurrying the initial product in 2-propanol at room temperature [100 mg/mL]). The mixture was cooled to room temperature at a rate of 5 oC per hour. After stirring at room temperature for 14 h, the mixture was filtered and dried to provide the final product as a white crystalline solid (136.84 g, 74%). 1H NMR (400 MHz, CDCIs): 7.88 - 7.78 (m, 1 .78H), 7.75 - 7.69 (s, 1 .22H), 7.51 - 7.43 (m, 1 H), 7.17 - 7.1 1 (m, 1 H), 6.30 - 6.28 (m, 1 H), 4.03 - 3.48 (m, 7H), 3.29 - 3.21 (m, 1 H), 3.15 - 2.92 (m, 2H), 2.30 (s, 6H). MS (ESI) mass calcd forC21 H22FN7O, 407.19; m/z found, 408 [M+H]+. Anal, calcd. for C21 H22FN7O C, 61 .90, H, 5.44, N, 24.06; found C, 61 .83, H, 5.42, N, 24.08
References:
Location in patent:Page/Page column 144-145
![3-Benzyl-3,7-diazabicyclo[3.3.0]octane](/CAS/GIF/86732-22-1.gif)
86732-22-1
100 suppliers
$40.00/100mg

1293281-49-8
42 suppliers
inquiry
![5-Benzyl-2-Boc-hexahydro-pyrrolo[3,4-C]pyrrole](/CAS/GIF/186202-73-3.gif)
186202-73-3
41 suppliers
$254.00/0.25G

1293281-49-8
42 suppliers
inquiry
![2-BOC-HEXAHYDRO-PYRROLO[3,4-C]PYRROLE](/CAS/GIF/141449-85-6.gif)
141449-85-6
143 suppliers
$24.00/250mg

1293281-49-8
42 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1293281-49-8
42 suppliers
inquiry