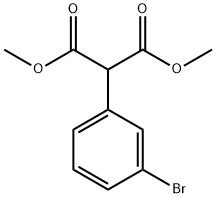
JXNAZEYUONMIGH-UHFFFAOYSA-N synthesis
- Product Name:JXNAZEYUONMIGH-UHFFFAOYSA-N
- CAS Number:773134-24-0
- Molecular formula:C11H11BrO4
- Molecular Weight:287.11
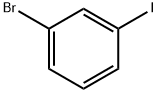
591-18-4
390 suppliers
$5.00/5g
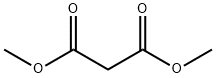
108-59-8
579 suppliers
$5.00/25g

773134-24-0
18 suppliers
$188.00/250mg
Yield:773134-24-0 70 g
Reaction Conditions:
with 2-Picolinic acid;copper(l) iodide;caesium carbonate in 1,4-dioxane at 60;
Steps:
1 Step 1. Dimethyl 2-(3-bromophenyl)malonate
2-picolinic acid (7.31 g, 59.4 mmol), Cul (5.65 g, 29.7 mmol), and CS2CO3 (161 g, 495 mmol) were added to a mixture of 1-bromo-3-iodo-benzene (31.5 mL , 247.43 mmol) and dimethyl propanedioate (30.6 mL_, 267 mmol) in nitrogen flushed dioxane (1 L). The mixture was stirred at 60 °C for 4.2 hrs. The solid was filtered off, and the filtrate was concentrated in vacuo. The mixture was diluted in aqueous NH4CI (5%, 300 mL) , and extracted with EA (300 mL x 2). The combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by FCC (Eluent: PE:EA = 20:1) affording the title compound (70 g) as light yellow oil. (1255) 1H NMR (400MHz, CDCI3) δ= 7.52 - 7.48 (m, 1 H), 7.44 - 7.37 (m, 1 H), 7.30 - 7.25 (m, 1 H), 7.18 - 7.12 (m, 1 H), 4.53 (s, 1 H), 3.69 (s, 6H) ppm.
References:
WO2021/155316,2021,A1 Location in patent:Page/Page column 363