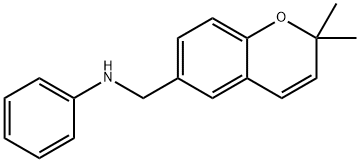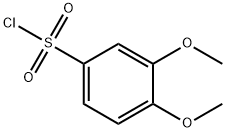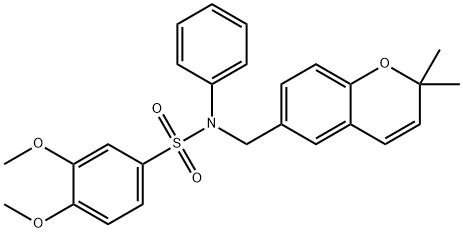
KCN1 synthesis
- Product Name:KCN1
- CAS Number:927823-01-6
- Molecular formula:C26H27NO5S
- Molecular Weight:465.56
Yield:927823-01-6 31%
Reaction Conditions:
with triethylamine in dichloromethane;Reflux;Inert atmosphere;
Steps:
4.1.5.10. 3,4-Dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide (6e)
General procedure: A mixture of 22 mg (0.093 mmole) of 4a, 0.1 mL (164 mg,0.93 mmole, 10 equiv) of benzenesulfonyl chloride, and 1 mL of triethylaminein 2 mL of methylene chloride was refluxed overnight.After cooling, the reaction mixture was concentrated in vacuo, andpurified by column chromatography using ethyl acetate/hexane(1:6) as an eluent to afford 9 mg (0.024 mmole) of N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-(propan-2-yl)benzenesulfonamide(5a, 26%). All 15 sulfonamides were synthesized by the samemethod.
References:
Mun, Jiyoung;Jabbar, Adnan Abdul;Devi, Narra Sarojini;Liu, Yuan;Van Meir, Erwin G.;Goodman, Mark M. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 14,p. 4590 - 4597] Location in patent:experimental part
69964-40-5
19 suppliers
inquiry

927823-01-6
2 suppliers
inquiry
![Benzaldehyde, 4-[(1,1-dimethyl-2-propyn-1-yl)oxy]-](/CAS/20210305/GIF/54730-31-3.gif)
54730-31-3
0 suppliers
inquiry

927823-01-6
2 suppliers
inquiry

123-08-0
899 suppliers
$5.00/10g

927823-01-6
2 suppliers
inquiry