
KIFUNENSINE DIACETONIDE synthesis
- Product Name:KIFUNENSINE DIACETONIDE
- CAS Number:134234-43-8
- Molecular formula:C14H20N2O6
- Molecular Weight:312.32
![D-Mannose, 5-[(aminooxoacetyl)amino]-5-deoxy-2,3:4,6-bis-O-(1-methylethylidene)- (9CI)](/CAS/20210305/GIF/128741-70-8.gif)
128741-70-8
1 suppliers
inquiry

134234-43-8
16 suppliers
inquiry
Yield:134234-43-8 306 mg
Reaction Conditions:
with ammonia in methanol;dichloromethane at 20;
Steps:
11 11. Synthesis of intermediate 14:
Intermediate 12 (0.5 g, 1.50 mmol) was dissolved in 5 mL dichloromethane (DCM)Then add 0.1g of 200-300 mesh silica gel powder, pyridinium dichromate salt (PDC, 1.13 g, 3.00 mmol),100 μl of trifluoroacetic acid (TFA) and 200 μl of pyridine (pyridine), the system is protected by argon gas and stirred at room temperature for 5 hours. The reaction was completed by TLC, and the system after the reaction was filtered. The filtrate was collected, and the solvent was removed under reduced pressure to give a reddish brown oily liquid. The crude product of Intermediate 13 was used in the next step without purification. Add 100 ml of 7N ammonia in methanol to the obtained reddish brown oily liquid. The reaction was stirred at room temperature overnight, and the reaction was complete by TLC. The solvent was removed under reduced pressure and the residue was extracted with ethyl acetate. After drying with anhydrous sodium sulfate, dried and concentrated under reduced pressure to remove solvent. The residue was purified on a silica gel column. Obtained 306 mg of a white solid. That is, intermediate 14.
References:
CN110256422,2019,A Location in patent:Paragraph 0087-0090
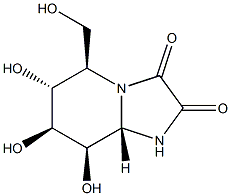
109944-15-2
78 suppliers
$50.00/2mg

77-76-9
386 suppliers
$9.00/5ml

134234-43-8
16 suppliers
inquiry

128741-75-3
19 suppliers
$55.00/5mg

134234-43-8
16 suppliers
inquiry
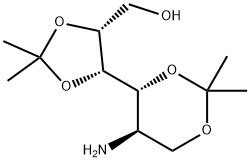
149819-14-7
1 suppliers
inquiry

134234-43-8
16 suppliers
inquiry
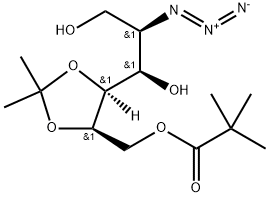
872038-08-9
1 suppliers
inquiry

134234-43-8
16 suppliers
inquiry