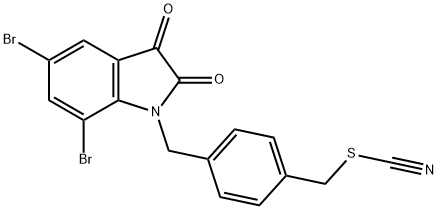
KS-99 synthesis
- Product Name:KS-99
- CAS Number:1344698-28-7
- Molecular formula:C17H10Br2N2O2S
- Molecular Weight:466.15
![1H-Indole-2,3-dione, 5,7-dibromo-1-[[4-(bromomethyl)phenyl]methyl]-](/CAS/20210305/GIF/1344698-26-5.gif)
1344698-26-5
0 suppliers
inquiry
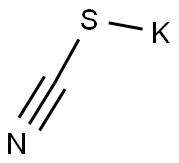
333-20-0
449 suppliers
$13.50/100G

1344698-28-7
4 suppliers
inquiry
Yield:1344698-28-7 74%
Reaction Conditions:
with potassium iodide in acetonitrile at 20;
Steps:
4.1.2. 5,7-Dibromo-N-(3’-thiocyanatopropyl)isatin (4)
General procedure: Compound 3 (200 mg, 0.52 mmol) was dissolved in anhydrous acetonitrile (15 mL), and KI (300 mg, 1.81 mmol) and KSCN (200 mg, 2.05 mmol) were added. The mixture was stirred overnight until a substantial amount of product was formed. The reaction mixture was diluted with water and extracted with ethyl acetate (3 × 50 mL). The organic layer was washed with water, dried over anhydrous MgSO4, and evaporated to dryness on the rotary evaporator. The crude product was purified by silica gel column chromatography (CH2Cl2-hexane, 7:3) eluted the product 4 as a yellow-orange solid (140 mg, 65%).
References:
Krishnegowda, Gowdahalli;Prakasha Gowda;Tagaram, Hephzibah Rani S.;Carroll, Kevin F. Staveley-O;Irby, Rosalyn B.;Sharma, Arun K.;Amin, Shantu [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 20,p. 6006 - 6014] Location in patent:experimental part
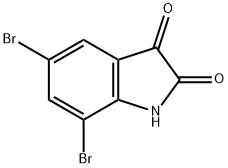
6374-91-0
56 suppliers
$25.00/250mg

1344698-28-7
4 suppliers
inquiry
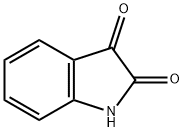
91-56-5
456 suppliers
$5.00/25g

1344698-28-7
4 suppliers
inquiry