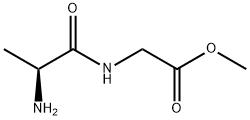
L-Alanylglycine methyl ester synthesis
- Product Name:L-Alanylglycine methyl ester
- CAS Number:51513-59-8
- Molecular formula:C6H12N2O3
- Molecular Weight:160.17
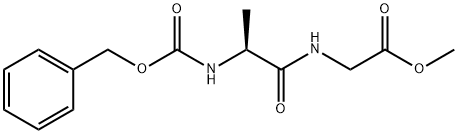
4840-29-3
20 suppliers
inquiry

51513-59-8
26 suppliers
$356.00/1g
Yield:-
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; under 2250.23 Torr; for 1 h;
Steps:
General procedure for preparation of piperazine-2,5-diones 3-5
General procedure: In a 3-neck round bottom flaskequipped with a septum, a thermometer and an adapter for a balloon with argon, Cbz-aminoacid(1.0 equiv., 5.0 mM) and N-ethylpiperidine (1.1 equiv., 5.5 mM) were dissolved in anhydrous methylenechloride (25 mL) and cooled to 10 C. Ethyl chloroformate (1.1 equiv., 5.5 mM) was added at the sametemperature, and the mixture protected from ingress of moisture was stirred for 2 h. Hydrochlorideof methyl L-alaninate or methyl glycinate (1.0 equiv., 5.0 mM) was dissolved in the second ask inanhydrous methylene chloride (10 mL) and cooled to 0 C, and then N-ethylpiperidine (1.0 equiv.,5.0 mM) was added. The whole volume of the second flask was immediately transferred to a 3-neckround bottom flask with the help of syringe, and additional N-ethylpiperidine (1.0 equiv., 5.0 mM)was added. The solution was stirred at the same temperature for 2 h and then left at 5 C overnight.After that, the reaction mixture was quenched with water (35 mL). The aqueous phase was extractedwith methylene chloride (3 15 mL). Combined organic phases were washed with 2M HCl solution(3 10 mL), water (10 mL), saturated sodium hydrogen carbonate solution (3 10 mL), and brine(10 mL) and dried over anhydrous sodium sulfate. After filtration, the solvent was removed underreduced pressure to give Cbz-amino dipeptide esters. The crude Cbz-amino dipeptide esters werehydrogenated with 60 mL of methanol and 10% Pd/C as a catalyst (0.5 g of Pd/C per 3 mM ofintermediate dipeptide esters) at room temperature. The reactor (250 mL capacity Parr reactor) waspressurized with hydrogen to 3 bars at ambient temperature. Reactor pressure remained unchangedduring the reaction period of 1 h. After releasing the pressure, the catalyst was removed by filtrationthrough Celite, and the solvent was removed under reduced pressure. The crude amino dipeptideesters were placed into a 250 mL round-bottomed flask equipped with an egg-shaped, Teflon-coated magnetic stirring bar and a reflux condenser, and toluene (100 mL) containing 5 mL of acetic acidwas added. The reaction mixture was stirred and refluxed for 2 h, then the reflux condenser wasremoved, and the amount of the solvent was reduced to 10% at atmospheric pressure. The remainingsolvent was removed under reduced pressure and the residue was recrystallized from propane-2-ol.Pure piperazine-2,5-diones 3-5 were dried at 40 C under reduced pressure. (3S)-3-Methylpiperazine-2,5-dione (3) [47] was prepared according to the general proceduredescribed above from [(benzyloxy)carbonyl]-L-alanine and hydrochloride of methyl glycinate.Yield 54%; Mp 278-280 C; []25D: 4.0 (CH3OH), 5 (H2O) [48] or 3.78 (H2O) [49]; IR (cm1): 3047,2879, 1663, 1456, 1327, 1094, 1044, 802, 669; 1H-NMR (DMSO-d6), : 8.09 (br. s., 1H), 7.90 (br. s., 1H),3.84 (dq, J = 0.9, 7.0 Hz, 1H), 3.78-3.67 (m, 2H), 1.26 (d, J = 7.0 Hz, 3H); 13C-NMR (DMSO-d6), : 168.8,166.2, 49.7, 44.5, 18.6; HR-MS: for C5H9N2O2 [M + H]+ calculated 129.0659 m/z, found 129.0652 m/z.
References:
Pokorna, Aneta;Bobal, Pavel;Oravec, Michal;Rarova, Lucie;Bobalova, Janette;Jampilek, Josef [Molecules,2019,vol. 24,# 3,art. no. 566]
![Glycine, N-[N-[(2-nitrophenyl)thio]-L-alanyl]-, methyl ester (9CI)](/CAS/20210305/GIF/7360-10-3.gif)
7360-10-3
0 suppliers
inquiry

51513-59-8
26 suppliers
$356.00/1g