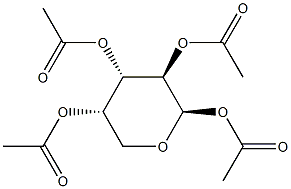
β-L-Arabinopyranose tetraacetate synthesis
- Product Name:β-L-Arabinopyranose tetraacetate
- CAS Number:4258-00-8
- Molecular formula:C13H18O9
- Molecular Weight:318.28
Yield:4258-00-8 126 g
Reaction Conditions:
with pyridine at 20;
Steps:
1 Example 1
50g of L-arabinose was stirred in 800mL of pyridine, and 270g of acetic anhydride was gradually added, and the system gradually became pale yellow and transparent. Then the reaction was stirred at room temperature overnight. After the reaction was completed, 100mL of methanol was added to quench the reaction, and then the system solvent was removed The remaining oil was dissolved in 500 ml of dichloromethane, washed with 200 mL of 1M hydrochloric acid, and then washed with 200 mL of saturated sodium bicarbonate, and then the organic phase was collected and dried over anhydrous sodium sulfate. After the drying was completed, the solvent was removed to obtain 126 g of a yellow oil, that is, Fully acetylated arabinose was dissolved in 300 mL of dichloromethane, 42 g of thiophenol and 55 g of 47% boron trifluoride ether solution were added in order. The system was stirred at room temperature for overnight reaction. After the reaction was completed, 50 g of triethylamine was added. After quenching the reaction, the system solvent was spun off, and the remaining oil was purified by a rapid silica gel column to obtain 115 g of a colorless oil, namely, sugar donor A1, with a yield of 94%.
References:
CN110590872,2019,A Location in patent:Paragraph 0037-0040
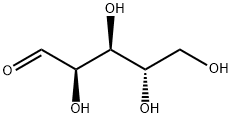
5328-37-0
462 suppliers
$5.00/1g
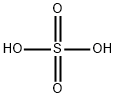
7664-93-9
6 suppliers
$23.30/1090731000
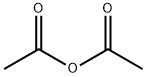
108-24-7
5 suppliers
$14.00/250ML

4258-00-8
3 suppliers
inquiry
