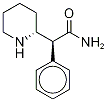
L-erythro-α-Phenyl- synthesis
- Product Name:L-erythro-α-Phenyl-
- CAS Number:160707-36-8
- Molecular formula:C13H18N2O
- Molecular Weight:218.29
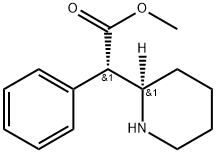
40431-63-8
1 suppliers
inquiry

160707-36-8
8 suppliers
inquiry
Yield:160707-36-8 75 %
Reaction Conditions:
Stage #1: methyl (αS,2R)-phenyl-(piperidin-2-yl)acetatewith hydrogenchloride in water;Reflux;
Stage #2: with thionyl chloride in dichloromethane at 0;Reflux;
Stage #3: with ammonia in tetrahydrofuran;methanol at 0 - 20;
Steps:
11 Example 11: Synthesis of (S)-2-phenyl-2-((R)-piperidin-2-yl)acetamide
Add 2a (466mg, 2.0mmol), 10mL water and 3mL concentrated hydrochloric acid into a 50mL reaction flask, and stir overnight under reflux.After the reaction system was cooled, the solvent was removed under reduced pressure to obtain a pale yellow crude product which was directly used in the next reaction.The crude product was dissolved in anhydrous dichloromethane in a 20mL reaction flask, and thionyl chloride was slowly added dropwise at 0°C.After stirring for half an hour, the reaction system was slowly heated to reflux and stirred for another 2 hours.After the solvent was removed under reduced pressure, the residue was dissolved in 12 mL of tetrahydrofuran, and then a 7M ammonia methanol solution was slowly added dropwise at 0° C., and the reaction system was raised to room temperature and then stirred for 2 hours.Quenched with water at 0 °C, added 3 mL of 5M hydrochloric acid solution and stirred for 1 hour.After extraction, the aqueous phase was collected, adjusted to pH 12 with 30% aqueous sodium hydroxide solution and extracted with ethyl acetate.Collect organic phase and remove solvent under reduced pressure, just can obtain product 3 (327mg, productive rate: 75%) through column chromatography
References:
CN115322141,2022,A Location in patent:Paragraph 0088-0092