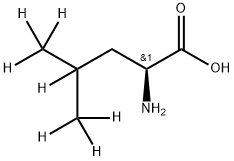
L-LEUCINE-D7 synthesis
- Product Name:L-LEUCINE-D7
- CAS Number:92751-17-2
- Molecular formula:C6H13NO2
- Molecular Weight:131.18
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate in 1,4-dioxane;water;
Steps:
Synthesis of Deuterium Labeled RGYALG
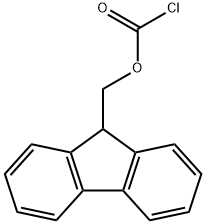
General procedure: Fmoc protected amino acids (e.g., α-d2-glycine, β-d2-tyrosine, etc.) were synthesized by reaction with 1× Fmoc-oSu and 1× NaHCO3 in 1:1 dioxane: H2O solution overnight. After dissolving reaction mixture in 5 % HCl, the product was taken up using ethyl acetate and washed with 0.1 MHCl and water. Following drying with anhydrous Na2SO4, organic solvent was evaporated away and the protected amino acid was crystallized. Peptides were synthesized bysolid phase methodology using Wang-resin and Fmoc chemistry. HCTU and piperidine was used for carboxylgroup activation and amine group deprotection, respectively, whereas DCB was used for adding the first residue to Wang resin. Peptides were cleaved from the resin with 95:2.5:2.5TFA:H2O:TIPS.
References:
Zhang, Xing;Julian, Ryan R. [Journal of the American Society for Mass Spectrometry,2013,vol. 24,# 4,p. 524 - 533]
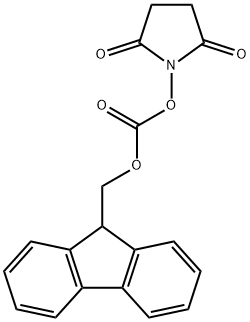
![L-Leucine-4,5,5,5,5',5',5'-d7, N-[(9H-fluoren-9-ylmethoxy)carbonyl]-](/CAS/20211123/GIF/1190594-50-3.gif)