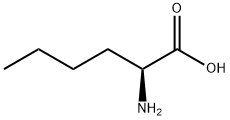
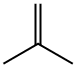
L-Norleucine tert-butyl ester synthesis
- Product Name:L-Norleucine tert-butyl ester
- CAS Number:15911-73-6
- Molecular formula:C10H21NO2
- Molecular Weight:187.28
Yield:15911-73-6 51%
Reaction Conditions:
with sodium hydroxide;sulfuric acid in 1,4-dioxane;
Steps:
1.2 (2)
(2) L-norleucine tert-butyl ester 2.0 g of L-norleucine was introduced into a pressure tube, and about 30 ml of dry dioxane was added thereto to obtain a suspension. Then, 2 ml of concentrated sulfuric acid was added thereto, and the reactor was cooled to -75° C. Then, isobutene gas was blown thereinto. When about 10 ml of isobutene gas was introduced, the tube was sealed. The temperature was gradually returned to room temperature under stirring, and then the stirring was continued overnight. Then, the reactor was opened, and isobutene was evaporated under atmospheric pressure. Then, the reaction solution was poured into 200 ml of a 2M sodium hydroxide aqueous solution under cooling with ice, and the mixture was vigorously stirred. Then, the mixture was extracted with diethyl ether (250 ml*2 times). The separated ether layer was washed with a saturated sodium chloride aqueous solution (100 ml*2 times) and then dried over anhydrous magnesium sulfate. The inorganic salt was filtered off, and the filtrate was concentrated to obtain a syrup. The syrup was thoroughly dried under reduced pressure and then stored at a temperature of not higher than 5° C., whereby 1.46 g (yield: 51%) of the above identified compound precipitated as white crystals. Rf: 0.71 (chloroform/methanol/conc.aqueous ammonia=(10/0.5/0.2). NMR (300 MHz, CDCl3).
References:
US5122523,1992,A