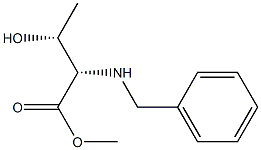
L-Threonine, N-(phenylmethyl)-, methyl ester synthesis
- Product Name:L-Threonine, N-(phenylmethyl)-, methyl ester
- CAS Number:196862-32-5
- Molecular formula:C12H17NO3
- Molecular Weight:223.2683

39994-75-7
283 suppliers
$6.00/1g

100-52-7
950 suppliers
$20.37/250g

196862-32-5
0 suppliers
inquiry
Yield:196862-32-5 52%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20;
Steps:
38.1 Step 1. Synthesis of Intermediate Compound 192
To a solution of compound 190 (5.3 g, 21.48 mmol) and compound 191 (2.1, 19.53 mmol) in DCM (20 mL), was added DIPEA (7.56 g, 58.58 mmol). The resulting mixture was stirred at room temperature overnight. Then to the mixture was added NaBH4 (0.82 g, 21.48 mmol). The resulting mixture was stirred for 4 h. The crude mixture was quenched with aq. NH4CI (50 mL) and extracted with ethyl acetate (200 mL x 2). The combined organic phase was washed with brine, dried over anhydrous Na2SC>4, filtrated and concentrated to dryness. The residue was purified by silica gel column (PE : EA = 10 : 1) to give title compound 192, (2S,3R)-methyl 2- (benzylamino)-3-hydroxybutanoate (2.5 g, 52% yield). LC-MS (LC method 1): m/z 224 (M+l)+.
References:
WO2017/189866,2017,A1 Location in patent:Paragraph 0231
3373-59-9
56 suppliers
$44.00/5g

196862-32-5
0 suppliers
inquiry