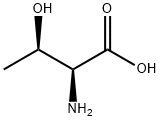
L-Threonine, N-(triphenylmethyl)- synthesis
- Product Name:L-Threonine, N-(triphenylmethyl)-
- CAS Number:80514-76-7
- Molecular formula:C23H23NO3
- Molecular Weight:361.43
Yield:80514-76-7 59%
Reaction Conditions:
Stage #1: L-threoninewith chloro-trimethyl-silane in dichloromethane; for 0.333333 h;Inert atmosphere;Reflux;
Stage #2: with triethylamine in dichloromethane at 0; for 1.08333 h;Inert atmosphere;
Stage #3: trityl chloridewith triethylamine in methanol;dichloromethane at 20; for 20 h;Inert atmosphere;
Steps:
4.2.1.12. N-Triphenylmethyl-(S)-serine (13).
General procedure: 4.2.1.12. N-Triphenylmethyl-(S)-serine (13). To a suspension of S-serine (2.0 g, 18 mmol) in DCM (26 ml) under a nitrogen atmosphere, was added Me3SiCl (7.5 ml, 57 mmol) and the solution was stirred at reflux temperature for 20 min. The solution was allowed to cool to 0 °C and Et3N (8.3 ml, 57 mmol) in DCM (26 ml) was added dropwise over 10 min to the solution, which was then stirred for 45 min. The solution was cooled to 0 °C and anhydrous methanol (0.9 ml) was added dropwise. The solution was allowed to warm to room temperature, and Et3N (7.0 ml, 18 mmol) was added followed by triphenylmethyl chloride (4.6 g, 18 mmol), and the solution was stirred for 20 h at ambient temperature. An excess of Et3N (13 ml) and then methanol (94 ml) was added until the white solid present in the mixture was dissolved. The excess solvent was then removed in vacuo producing a white solid. The white solid was partitioned between ethyl acetate (67 ml) and citric acid (40.2 ml, 5% aq w/v), which had been pre-cooled to 4 °C. The organic layer was washed with 2 M aqueous sodium hydroxide (220 ml) followed by water (315 ml). The aqueous layers were combined, washed with ethyl acetate (15 ml) and neutralised with glacial acetic acid (3e4 ml) at 0 °C. The precipitated product was extracted with ethyl acetate (630 ml) and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed in vacuoto produce a white solid (3.1 g, 48%).
References:
O'Brien, Keith;ó Proinsias, Keith;Kelleher, Fintan [Tetrahedron,2014,vol. 70,# 34,p. 5082 - 5092]