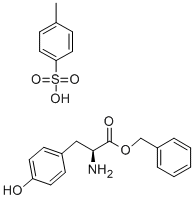
L-Tyrosine benzyl ester p-toluenesulfonate synthesis
- Product Name:L-Tyrosine benzyl ester p-toluenesulfonate
- CAS Number:53587-11-4
- Molecular formula:C23H25NO6S
- Molecular Weight:443.51
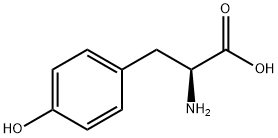
60-18-4
817 suppliers
$15.00/25g
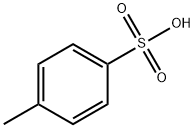
104-15-4
720 suppliers
$133.00/500g

100-51-6
1386 suppliers
$5.00/100g

53587-11-4
184 suppliers
$6.00/1g
Yield:53587-11-4 92%
Reaction Conditions:
in cyclohexane;water; for 4 h;Dean-Stark;Reflux;
Steps:
General procedure for the preparation of 1a-9a
General procedure: The esterifications were carried out on L amino acids withthe exception of phenylglycine, the D enantiomer of whichwas used. A mixture of amino acid (0.05 mol), p-toluenesulfonicacid (0.06 mol), benzyl alcohol (0.25 mol) andcyclohexane (30 mL) was refluxed for 4 h using a Dean-Stark apparatus to separate water that was azeotroped outas it formed. The reaction mixture was cooled to roomtemperature and ethyl acetate (80 mL) was added. Afterstirring for 1 h, the precipitate was collected by filtrationand dried to give the corresponding benzyl ester p-toluenesulfonateas a white solid. According to this procedure,the amino acids 1-6 were converted into the correspondingbenzyl ester p-toluenesulfonates 1a-6a. The benzylationof 7 was accomplished in the same manner but in thepresence of more p-toluenesulfonic acid (0.11 mol) to givethe di-p-toluenesulfonate 7a as a white solid. The p-toluenesulfonate8a separated at the end of the reaction as anoil; instead of adding ethyl acetate, the supernatant wasremoved, the oily phase was washed with cyclohexane andthen poured into dichloromethane/aqueous Na2CO3. Afterremoving the water layer and evaporating dichloromethane,the residue was treated with hydrochloric methanol to give the corresponding hydrochloride as a white solid. Thebenzylation of 9 was prolonged over night and, at the endof the reaction, 9a separated as an oil, which was pouredinto dichloromethane/water. After removing the organiclayer, the water phase was made alkaline with NaHCO3 andextracted with ethyl acetate. The organic extract was concentratedto a small volume and a slight excess of p-toluenesulfonicacid was added to precipitate 9a as a white crystallinesolid.
References:
Bolchi, Cristiano;Bavo, Francesco;Pallavicini, Marco [Amino Acids,2017,vol. 49,# 5,p. 965 - 974]

60-18-4
817 suppliers
$15.00/25g

100-51-6
1386 suppliers
$5.00/100g

53587-11-4
184 suppliers
$6.00/1g
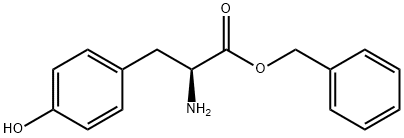
42406-77-9
56 suppliers
$20.16/1G

104-15-4
720 suppliers
$133.00/500g

53587-11-4
184 suppliers
$6.00/1g