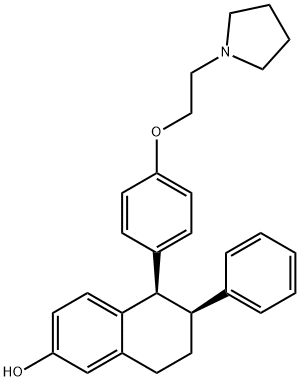
Lasofoxifene synthesis
- Product Name:Lasofoxifene
- CAS Number:180916-16-9
- Molecular formula:C28H31NO2
- Molecular Weight:413.55
![Pyrrolidine, 1-[2-[4-(1,2,3,4-tetrahydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy]ethyl]-](/CAS/20210305/GIF/5879-98-1.gif)
5879-98-1
0 suppliers
inquiry

180916-16-9
71 suppliers
$107.00/1mg
Yield:180916-16-9 80%
Reaction Conditions:
with boron tribromide in dichloromethane at -50 - 0; for 3 h;
Steps:
Lasofoxifene: (2) (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol)
To a solution of methyl ether 9 (600 mg, 1.4 mmol), in DCM (5 mL) added BBr3 in DCM(1.0 M, 8 mL) solution at -50 °C for 5 min. The reaction mixture was stirred for 3 h at 0 °C.After completion of the reaction (monitored by TLC), reaction mixture was poured into saturated sodium bicarbonate solution (25 mL) at 0°C. Then extracted with DCM (2X20 mL) and the organic layer was washed with brine solution (20mL). The organic layer dried over sodium sulfate and concentrated under vacuum to get the crude, crude crystallization in ethyl acetate toget the desired product 2 (Lasofoxifene). Yield: 430 mg (80%); Description: brown color solid;Mp: 132-134.3 °C; IR (in KBr, cm-1): 3471, 2928, 1775, 1652, 1503, 1177, 757; 1H NMR (400MHz, CDCl3) ( ppm): 7.16-7.14 (m, 3H, Ar), 6.80-6.78 (m, 3H, Ar), 6.66 (s, 1H, Ar), 6.57 (dd,J=8.1, 2.4 Hz, 1H, Ar), 6.45 (d, J=8.32 Hz, 2H, Ar), 6.27 (d, J=8.32 Hz, 2H, Ar), 4.21 (d, J=4.56Hz, 1H), 3.98 (m, 2H, OCH2), 3.35 (ddd, J=11.4, 4.8, 2.0 Hz, 1H), 2.99-2.97 (m, 2H), 2.94-2.91(m, 2H), 2.81 (m, 4H, Pyrrolidine), 2.13 (m, 1H), 1.82-1.76(m, 5H); 13C NMR (100 MHz,CDCl3) ( ppm): 156.6, 155.2, 144.8, 137.6, 134.6, 131.8, 131.4, 128.4, 128.2, 127.5, 126.0,114.7, 113.9, 112.6, 65.8, 55.2, 54.2, 50.8, 45.8, 30.0, 23.5, 21.9.
References:
Umareddy, Pailla;Arava, Veera Reddy [Synthetic Communications,2016,vol. 46,# 4,p. 309 - 313] Location in patent:supporting information