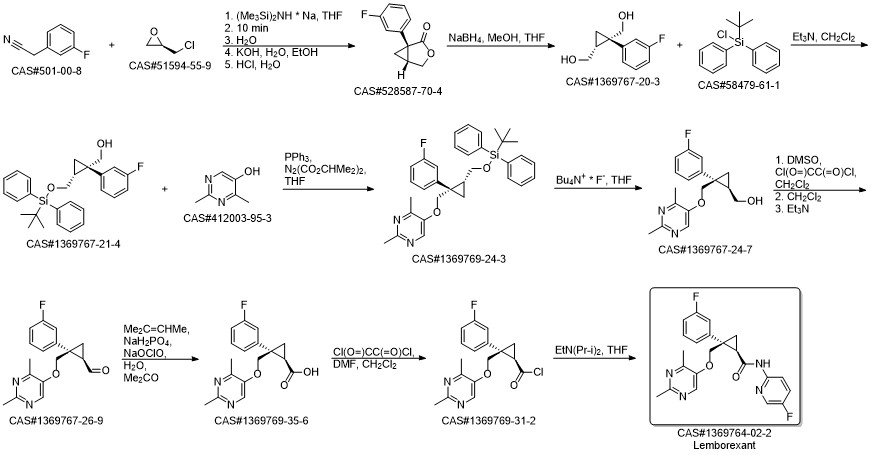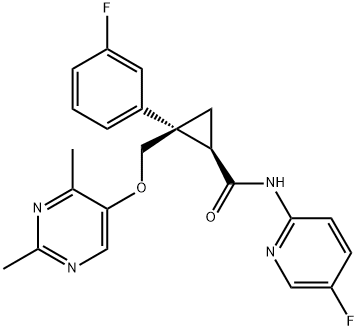
Lemborexant synthesis
- Product Name:Lemborexant
- CAS Number:1369764-02-2
- Molecular formula:C22H20F2N4O2
- Molecular Weight:410.42
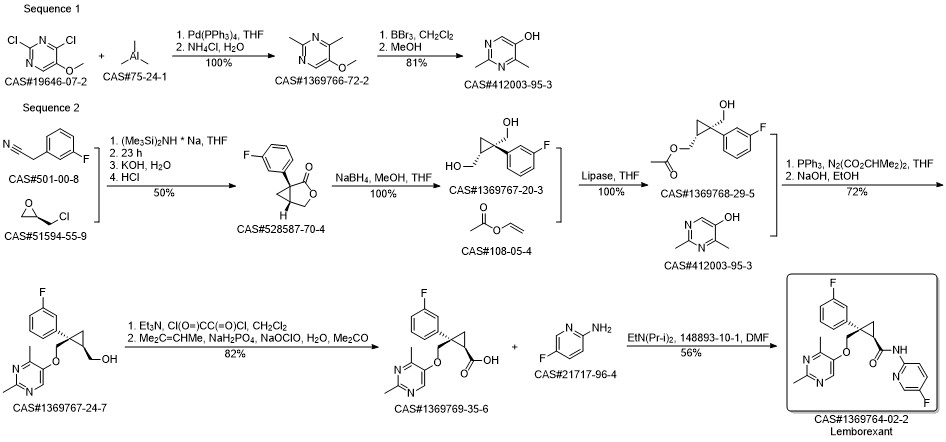
Yoshida, Yu; Naoe, Yoshimitsu; Terauchi, Taro; Ozaki, Fumihiro; Doko, Takashi; Takemura, Ayumi; Tanaka, Toshiaki; Sorimachi, Keiichi; Beuckmann, Carsten T.; Suzuki, Michiyuki; Ueno, Takashi; Ozaki, Shunsuke; Yonaga, Masahiro. Discovery of (1R,2S)-2-{[(2,4-Dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): A Potent and Efficacious Oral Orexin Receptor Antagonist. Journal of Medicinal Chemistry. Volume 58. Issue 11. Pages 4648-4664. Journal. (2015).
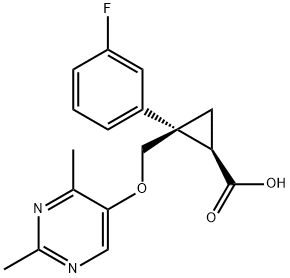
1369769-35-6
6 suppliers
inquiry
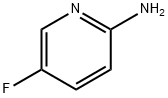
21717-96-4
409 suppliers
$10.00/1g

1369764-02-2
117 suppliers
inquiry
Yield:1369764-02-2 87%
Reaction Conditions:
with 1-propanephosphonic acid cyclic anhydride;N-ethyl-N,N-diisopropylamine in ethyl acetate at 70;Large scale;
Steps:
D (D) Production of (1R,2S)-2-(((2,4-dimethylpyrimidin-5-yl)oxy)methyl)-2-(3-fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (Lemborexant: Compound of Formula XI)
A mixture of (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3-fluorophenyl)cyclopropane carboxylic acid (62.4 kg, 197 mol, 1.00 equiv.), 5-fluoropyrimidin-2-amine (24.3 kg, 217 mol, 1.10 equiv.), ethyl acetate (499 L), N,N-diisopropylethylamine (53.5 kg, 414 mol, 2.10 equiv.) and 1-propanephosphoric acid anhydride (50% ethyl acetate solution) (188 kg, 296 mol, 1.50 equiv.) was heated at an external temperature of 70° C., and the end of a reaction was confirmed by HPLC analysis.
After a reaction liquid was cooled, 312 L of purified water was added thereto, stirred and then left to stand.
After a water layer was discharged, a sodium carbonate aqueous solution (68.9 kg of sodium carbonate and 312 L of water) was added to an organic layer, stirred and then left to stand.
After the water layer was discharged, purified water (187 L) was added to the organic layer, stirred and then left to stand.
The water layer was discharged, and purified water (187 L) was added to the organic layer, stirred and then left to stand.
The water layer was discharged, and the organic layer was filtered.
A clarification filtration line was rinsed with ethyl acetate, and the solvent was then partially distilled away under reduced pressure.
A mixture prepared by adding ethyl acetate to a concentrated residue (containing substantially 75.3 kg of lemborexant) such that the total volume reached 256 L was heated and dissolved under stirring at an external temperature of 60° C. and then cooled to 45° C. or lower by adding n-heptane (12.8 kg) thereto.
Ethyl acetate (31 L) was added thereto, the mixture was cooled to 35° C. or lower, and then n-heptane (670 kg) was added thereto.
After that, a suspension was cooled to 10° C. or lower, and a solid in the mixture was filtered and washed with a liquid mixture of ethyl acetate and n-heptane.
The obtained solid was dried at an external temperature of 60° C. under reduced pressure, thereby obtaining lemborexant (70 kg) in a yield of 87%.
References:
US2022/194925,2022,A1 Location in patent:Paragraph 0334-0346
![Cyclopropanecarbonyl chloride, 2-[[(2,4-dimethyl-5-pyrimidinyl)oxy]methyl]-2-(3-fluorophenyl)-, (1R,2S)-](/CAS/20210305/GIF/1369769-31-2.gif)
1369769-31-2
1 suppliers
inquiry

21717-96-4
409 suppliers
$10.00/1g

1369764-02-2
117 suppliers
inquiry

1369769-35-6
6 suppliers
inquiry

1369764-02-2
117 suppliers
inquiry
![(1S,5R)-1-(3-fluorophenyl)-3-oxabicyclo[3.1.0]hexan-2-one](/CAS/20200119/GIF/528587-70-4.gif)
528587-70-4
21 suppliers
inquiry

1369764-02-2
117 suppliers
inquiry
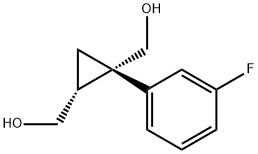
1369767-20-3
20 suppliers
inquiry

1369764-02-2
117 suppliers
inquiry