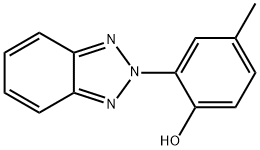
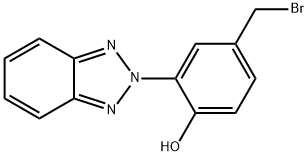
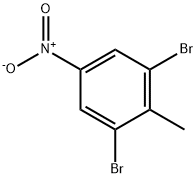
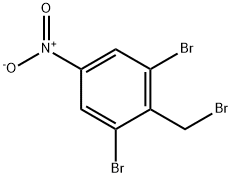
Lenalidomide Impurity 13 synthesis
- Product Name:Lenalidomide Impurity 13
- CAS Number:764-28-3
- Molecular formula:C8H12N4
- Molecular Weight:164.21
Yield:478148-82-2 89%
Reaction Conditions:
with tri-n-butyl-tin hydride in benzene;
Steps:
6.v.i EXAMPLE 7(i)
C50 (5.51 g, 16.9 mmol) is suspended in benzene (30 mL) in a dry flask under N2. Azo(bis)isobutyryl nitrile (289 mg, 1.8 mmol) is added, the mixture is rapidly heated to reflux, and tributyltin hydride (4.91 mL, 18.2 mmol) in benzene (10 mL) is added. The solution is refluxed for 1.5 h, allowed to cool to rt and concentrated in vacuo. The resulting residue is chromatographed over 125 g slurry-packed silica gel, eluding with a gradient of EtOAc/hexane (20%-60%) to afford (7-chloro-3-methyl-2,3-dihydrofuro[2,3-c]pyridin-5-yl)methanol (C51) as a white solid (89% yield). MS (ESI) for C9H10ClNO2+H, m/z: 200.1 (M+H).
References:
US2003/153595,2003,A1

764-28-3
5 suppliers
inquiry
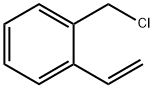
22570-84-9
8 suppliers
inquiry

![Furo[2,3-c]pyridine-5-methanol, 7-chloro-2,3-dihydro-3-methyl-](/CAS/20210305/GIF/478148-82-2.gif)