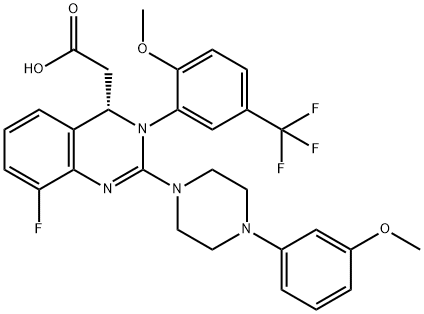
LeterMovir synthesis
- Product Name:LeterMovir
- CAS Number:917389-32-3
- Molecular formula:C29H28F4N4O4
- Molecular Weight:572.55

Reference: Humphrey, Guy & M. Dalby, Stephen & Andreani, Teresa & Xiang, Bangping & R. Luzung, Michael & Jake Song, Zhiguo & Shevlin, Michael & Christensen, Melodie & Belyk, Kevin & M. Tschaen, David. (2016). Asymmetric Synthesis of Letermovir Using a Novel Phase-Transfer-Catalyzed Aza-Michael Reaction. Organic Process Research & Development. 20. 10.1021/acs.oprd.6b00076.
![Butanedioic acid, 2,3-bis[(4-methylbenzoyl)oxy]-, (2S,3S)-, compd. with methyl (4S)-8-fluoro-3,4-dihydro-2-[4-(3-methoxyphenyl)-1-piperazinyl]-3-[2-methoxy-5-(trifluoromethyl)phenyl]-4-quinazolineacetate (1:1)](/CAS/20180808/GIF/917389-30-1.gif)
917389-30-1
15 suppliers
inquiry

917389-32-3
186 suppliers
$15.00/1mg
Yield:917389-32-3 96.4%
Reaction Conditions:
with water;sodium hydrogencarbonate;sodium hydroxide in tert-butyl methyl ether;Reflux;Large scale;
Steps:
3b
A mixture of (2S 3^-2,3-bis [(4-methylbenzoyl)oxy]succininc acid -{(45 8-fluoro-2-[4-(3- methoxyphenyl)piperazin- 1 -yl] -3 -[2-methoxy-5-(trifluoromethyl)phenyl] -3 ,4-dihydro- quinazolin-4-yl} acetic acid methyl ester (l :l-salt) (30,8 kg), sodium hydrogen carbonate (16.4 kg) and water (315 L) is stirred with MTBE (160 L). The phases obtained are separated and the organic phase is treated with 35 L of a 7% sodium hydrogen carbonate solution. The phases obtained are separated again and the organic phase is treated with 125 L of a 4% sodium hydroxide solution. The mixture is heated under reflux conditions. The solvent is distilled to run dry. The residual content of the reactor is stirred for further 5 h at 55 - 60°C. To the mixture MTBE (160 L) and water (65 L) is added under stirring at 22°C. The phases obtained are separated again and the organic phase is extracted with the aid of a 6% aqueous sodium chloride solution (30 L). The aqueous phases are reunited and stirred with water (25 L) and MTBE (160 L). The pH is adjusted to 6.5 with the aid of IN muriatic acid. The organic phase is separated, the solvent is gently distilled to run dry and the residue is dissolved in acetone (approximately 75 L). A change of the solvent is conducted towards acetone by means of 6 distillation steps of 130 L each. The product is subsequently precipitated by adding the residual solvent (approximately 60 L) under stirring conditions (61 rpm) in an excess of water (492 L) at room temperature. Followed by centrifugation, the isolated product is dried in a vacuum dryer equipped with a spiral crumbling roller at 40 to 80°C. By this procedure a yield of 16,5 kg of (S)-{8-fluoro-2-[4-(3-methoxyphenyl)piperazin- l-yl]-3-(2-memoxy-5-trifluormethylphenyl)-3,4-dihydroquina2olin-4-yl}acetic acid is obtained as amorphous compound corresponding to 96.4% in theory.1H NMR (300 MHz, d6-DMSO): δ = 7,53 (d,2J - 8,4, 1H), 7,41 (brs, 1H), 7,22 (d,2J = 8,5, 1H), 7,09-7,01 (m, 2H), 6,86 (m, 2H)56,45 (dd,2J = 8,2,3J = 1,8, 1H), 6,39-6,34 (m, 2H), 4,87 (t,2J= 7,3, 1H), 3,79 (brs, 3H), 3,68 (s, 3H), 3,50-3,38 (m, 4H), 2,96-2,75 (m, 5H), 2,45- 2,40 (m, 1H) ppm; MS (API-ES-neg.): m/z = 571 [(M-H), 100 %];
References:
WO2014/202737,2014,A1 Location in patent:Page/Page column 53
![4-Quinazolineacetic acid, 8-fluoro-3,4-dihydro-2-[4-(3-Methoxyphenyl)-1-piperazinyl]-3-[2-Methoxy-5-(trifluoroMethyl)phenyl]-, Methyl ester](/CAS/20150408/GIF/791117-40-3.gif)
791117-40-3
19 suppliers
inquiry

917389-37-8
20 suppliers
inquiry

917389-32-3
186 suppliers
$15.00/1mg
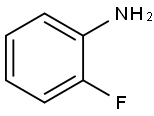
348-54-9
411 suppliers
$10.00/1g

917389-32-3
186 suppliers
$15.00/1mg
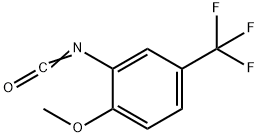
16588-75-3
85 suppliers
inquiry

917389-32-3
186 suppliers
$15.00/1mg
![N-(2-fluorophenyl)-N'-[2-methoxy-5-(trifluoromethyl)phenyl]urea](/CAS/20180702/GIF/917389-24-3.gif)
917389-24-3
15 suppliers
inquiry

917389-32-3
186 suppliers
$15.00/1mg






