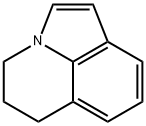
Lilolidine synthesis
- Product Name:Lilolidine
- CAS Number:5840-01-7
- Molecular formula:C11H11N
- Molecular Weight:157.21
![4H-PYRROLO[3,2,1-IJ]QUINOLINE-1-CARBOXYLIC ACID,5,6-DIHYDRO-](/CAS/GIF/124730-56-9.gif)
124730-56-9
59 suppliers
inquiry

5840-01-7
129 suppliers
$10.00/250mg
Yield:5840-01-7 72%
Reaction Conditions:
with quinoline;copper chromite at 185; for 2 h;
Steps:
1.4
5,6-dihydro-4H-pyrrolo [3,2,1-z/] quinoline-1-carboxylic acid (37.5 g, 0.186 mol), copper chromite (13.5 g, 43 mmol) and quinoline (180 ml) were heated with stirring to 1850C for 2 hours. The mixture was cooled, diluted with dichloromethane (1 L) and filtered over hyflo. The filtrate was washed with 2 M hydrochloric acid (2x600 ml) and twice with 2 M sodium hydroxide (150 ml) before being evaporated to dryness. The residue was purified by silica gel chromatography, eluting with a ethyl acetate/hexanes (1:6) to afford 5, 6-dihydro-4H-pyrrolo [3,2,1-z/] quinoline (21 g, 72%) as a pale yellow solid. 1H NMR (CDCl3) 400 MHz ?: 7.44(dd, IH, J=0.8 and 7.6 Hz), 7.07(d, IH, J=3.2 Hz), 7.01(t, IH, J=7.2 Hz), 6.9 (dd, IH, J=0.8 and 6.8 Hz), 6.43(d, IH, J=3.2 Hz), 4.16(t, 2H, J=6 Hz), 2.99(t, 2H, J=6.4 Hz), 2.24(m, 2H).
References:
ARQULE, INC. WO2006/86484, 2006, A1 Location in patent:Page/Page column 52
![1, 2, 3, 4, 5, 6-HEXAHYDROPYRROLO [3,2,1-I,J] QUINOLONE-2](/CAS/GIF/16078-37-8.gif)
16078-37-8
12 suppliers
inquiry

5840-01-7
129 suppliers
$10.00/250mg
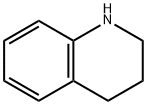
635-46-1
359 suppliers
$5.00/10g
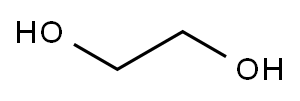
107-21-1
1334 suppliers
$10.00/25g

5840-01-7
129 suppliers
$10.00/250mg
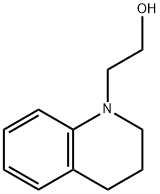
52704-48-0
12 suppliers
inquiry

635-46-1
359 suppliers
$5.00/10g

5840-01-7
129 suppliers
$10.00/250mg
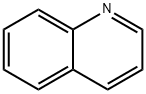
91-22-5
393 suppliers
$10.00/25g

107-21-1
1334 suppliers
$10.00/25g

635-46-1
359 suppliers
$5.00/10g

5840-01-7
129 suppliers
$10.00/250mg